Blood separation gel used for blood collection vessel and preparation method thereof
A blood separation and blood collection technology, applied in the direction of immiscible liquid separation, instruments, analysis materials, etc., can solve the problems of compatibility difference, loss of separation and barrier effect, and influence of test results, etc., to achieve good compatibility, Effects of improving physical characteristics and reducing detection errors and difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
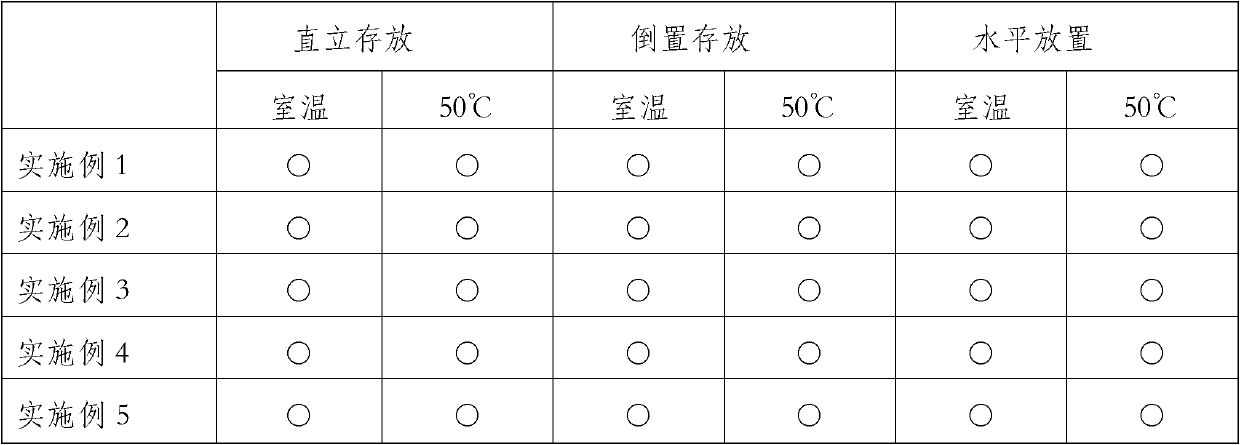
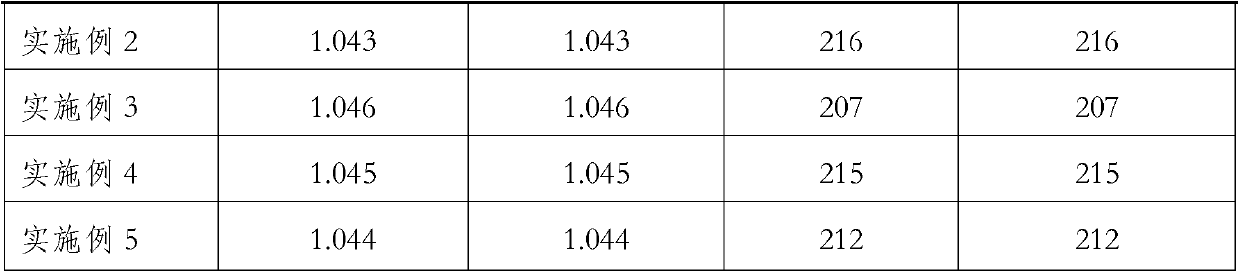
Embodiment 1
[0022] Weigh 26 parts by weight of chlorinated paraffin (52% degree of chlorination, specific gravity at 25°C is 1.255), 1 part by weight of dibenzylidene sorbitol, 65 parts by weight of polyisobutene (molecular weight 40000, specific gravity at 25°C is 0.92, Viscosity at 100°C is 30000mPa·s), put it into the planetary mixer, close the cover, stir while heating up, when the temperature rises to 120°C, keep constant temperature, continue stirring for 1 hour, so that all components are fully dissolved and mixed; cool to Below 60°C, add 8 parts by weight of nano-silica (hydrophobic fumed silica after dimethylpolysiloxane post-treatment, particle size 12nm, specific surface area 110m2 / g, compacted density about 50g / l ), close the lid, decompress and evacuate, and control the negative pressure at -0.09Mpa to continue stirring for 1 hour. After cooling to room temperature, take out the prepared blood separation gel.
Embodiment 2
[0024] Take by weighing 29 parts by weight of chlorinated paraffin (chlorination degree is 70%, specific gravity is 1.255 at 25 DEG C), 0.81 parts by weight of dibenzylidene sorbitol, 60 parts by weight of polyisobutylene (molecular weight 950, specific gravity at 25 DEG C is 0.89, Viscosity at 40°C is 1.6Pa·s) into the planetary mixer, close the lid, and stir while heating up. When the temperature rises to 120°C, keep constant temperature and continue stirring for 1 hour to fully dissolve and mix the components; cool to 60°C Below ℃, add 10.19 parts by weight of nano-silica (hydrophobic fumed silica after dimethyl polysiloxane treatment, particle size 12nm, specific surface area 110m2 / g, compacted density about 50g / l) Close the lid, depressurize and evacuate, control the negative pressure at -0.09Mpa and continue stirring for 1 hour. After cooling to room temperature, take out the prepared blood separation gel.
Embodiment 3
[0026] Take by weighing 20 parts by weight of chlorinated paraffin (52% in degree of chlorination, specific gravity at 25°C is 1.255, viscosity 1.6Pa·s), 0.8 parts by weight of dibenzylidene sorbitol, 65 parts by weight of polyisobutene (molecular weight 850, The specific gravity is 0.88 at 25°C, the viscosity is 1.4Pa s) into the planetary mixer, close the lid, and stir while heating up. When the temperature rises to 120°C, keep constant temperature and continue stirring for 1 hour to fully dissolve and mix the components. be cooled to below 60°C, add 14.2 parts of nano-silica (hydrophobic fumed silica after dimethylpolysiloxane post-treatment, particle diameter 12nm, specific surface area 110m2 / g, compacted density approx. 50g / l) and close the lid, decompress and vacuumize, keep the negative pressure at -0.09Mpa and continue to stir for 1 hour. After cooling to room temperature, take out the prepared blood separation gel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com