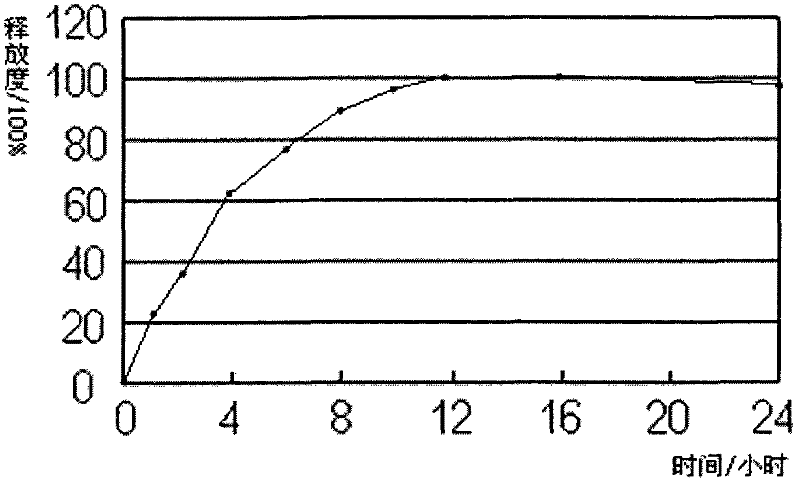
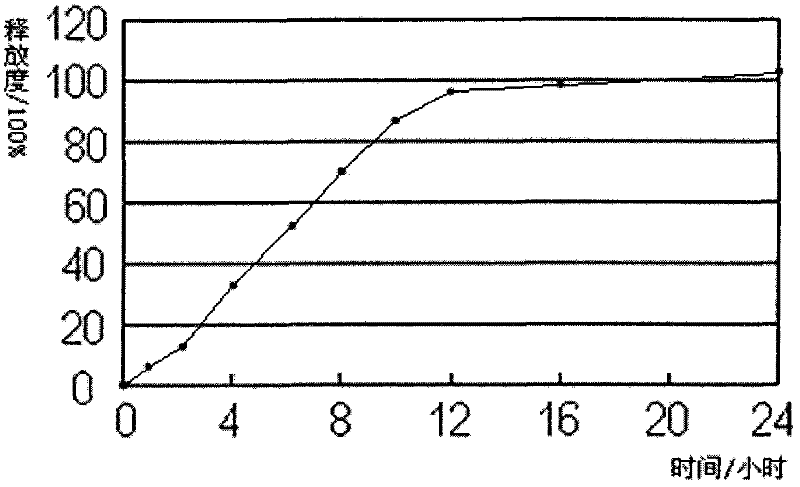
Oral quetiapine sustained-release tablet
A quetiapine and gentle technology, applied in the direction of medical preparations of non-active ingredients, organic active ingredients, nervous system diseases, etc., can solve the problems of low affinity and no affinity, so as to reduce drug resistance, increase release time, The effect of reducing volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Each 1000 tablets of quetiapine fumarate sustained-release tablets (200mg) contains:
[0019]
[0020] Prepared according to the following process:
[0021] (1) 230.26g of quetiapine fumarate, 32.32g of pregelatinized starch, 43.42g of microcrystalline cellulose, 8g of hypromellose and 75g of sodium citrate dihydrate were crushed and sieved as required, and mixed evenly , use 50% ethanol to make a suitable soft material, and granulate;
[0022] (2) Dry the wet granules, granulate, add 140g of polyoxyethylene N-12, 35g of polyoxyethylene N-60, 7g of magnesium stearate, 5g of stearic acid, 4g of talcum powder auxiliary materials and mix evenly during granulation.
[0023] (3) Tablets.
[0024] (4) Coating.
Embodiment 2
[0026] Each 1000 tablets of quetiapine fumarate sustained-release tablets (200mg) contains:
[0027]
[0028] Prepared according to the following process:
[0029] (1) 230.26g of quetiapine fumarate, 34.32g of pregelatinized starch, 34.32g of microcrystalline cellulose pH101, 75g of sodium citrate dihydrate and 8g of hypromellose E50 were crushed and sieved as required, Mix evenly, use 66g 50% ethanol to make a suitable soft material, and granulate;
[0030] (2) Dry the wet granules, granulate, add 124.1g of polyoxyethylene 1105, 60g of polyoxyethylene 303, 7g of magnesium stearate, 7g of silicon dioxide auxiliary materials and mix well during granulation.
[0031] (3) Shaped stamping sheet.
[0032] (4) Coating.
Embodiment 3
[0034] Each 1000 tablets of Quetiapine Fumarate Sustained Release Tablets (50mg) contains:
[0035]
[0036]
[0037] Prepared according to the following process:
[0038] (1) 57.6g of quetiapine fumarate, 60g of pregelatinized starch, 143.4g of microcrystalline cellulose, 36g of sodium citrate dihydrate and 8g of hypromellose E50 were crushed and sieved as required, and mixed evenly , use 60g 50% ethanol to make a suitable soft material, and granulate;
[0039] (2) Dry the wet granules, granulate, add 150g of polyoxyethylene 750, 35g of polyoxyethylene N-60, 5g of magnesium stearate, 5g of silicon dioxide auxiliary materials and mix evenly during granulation.
[0040] (3) Shaped stamping sheet.
[0041] (4) Coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com