A New Process for the Preparation of p-Toluenesulfonyl Chloride
A technology of methylbenzenesulfonyl chloride and a new process is applied in the field of pesticides to achieve the effects of improving reaction speed and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
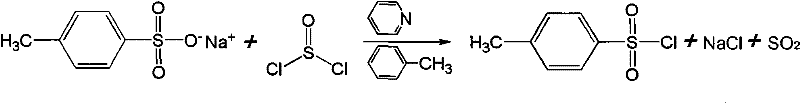
[0006] Embodiment one: a new preparation process of p-toluenesulfonyl chloride, the process uses crude product sodium p-toluenesulfonate as the main raw material to prepare p-toluenesulfonyl chloride through chlorination reaction, and the reaction formula is as follows:
[0007]
[0008] In a 500 ml four-necked flask equipped with a constant pressure funnel, a stirrer and a condenser, add 19.5 grams (100 percent) of crude product sodium p-toluenesulfonate, add 300 ml of toluene, add 1 ml of pyridine, and stir well Afterwards, the temperature was raised to reflux, and 18 grams of thionyl chloride began to be added dropwise from the liquid surface. After dropping, the reflux reaction was continued, and stirring was continued for 1 hour after the reaction solution became clear. After the end of the reaction, change it to a vacuum distillation device, and steam out toluene and pyridine under reduced pressure from the reaction solution (the solvent that is steamed out is directly...
Embodiment 2
[0009] Embodiment two: in the 500 milliliters of four-neck flasks that constant pressure funnel, agitator and condensing pipe are equipped with, add the crude product sodium p-toluenesulfonate of 19.5 grams (100 percent), add solvent 300 milliliters (embodiment one recovery), after stirring evenly, the temperature was raised to reflux, and 17 grams of thionyl chloride began to be added dropwise from the liquid surface. After dropping, the reflux reaction was continued, and the reaction solution continued to stir for 1 hour after the reaction solution became clear. After the reaction is over, change it to a vacuum distillation device, and steam out toluene and pyridine under reduced pressure from the reaction solution (the solvent that is steamed out is directly applied mechanically next time), wash with ice water, and dry to obtain 17.8 grams of product. Sodium acid calculated yield was 93.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com