The preparation method of 2,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and pentafluoropropane, which is applied in the field of preparation of a substitute for refrigerant HFC-134a, can solve the problems of harsh reaction conditions, use of toxic organic solvents, and low yield, and achieves mild reaction conditions and high reaction efficiency. Ease of operation and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation method of embodiment 1,2,3,3,3-tetrafluoropropene (HFO-1234yf)
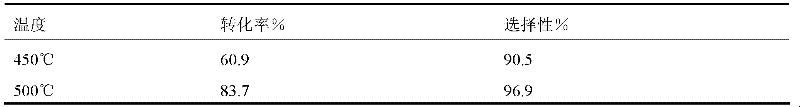
[0045] (1) Fill the Ni / C catalyst in a tubular reactor with an inner diameter of 2 cm and a length of 40 cm, first fill it with nitrogen, then heat it with steam to 450 ° C, and after passing nitrogen for one hour, pass CHCl 2 CF 2 CF 3 and H 2 Flow through the tube at a flow rate of 30cc / s, then raise the temperature to 500°C, and perform gas chromatographic analysis on the gas samples at the outlet of the reaction tube at the two temperatures. The obtained results are listed in Table 1.
[0046] (2) Using a mixture of fluorinated alumina and chromium fluoride as a catalyst to prepare HFO-1234yf by deHF reaction:
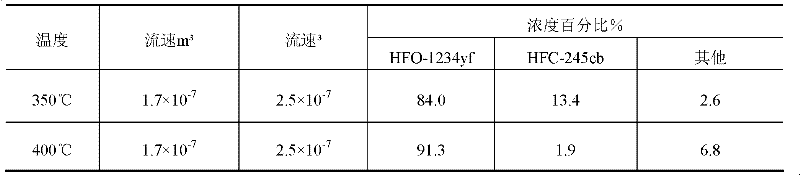
[0047] Catalyst preparation: Fill the solids of 15-20 mesh alumina and 15-20 mesh chromium fluoride in a molar ratio of 1:1 into a tubular reactor, which is heated by steam outside the tubular reactor at 250°C The catalyst was dried by heating under nitrogen purge for 20 mi...
Embodiment 2
[0058] Example 2. Phase studies of mixtures of HF and HFO-1234yf
[0059] A phase study was performed on a composition consisting essentially of HF and HFO-1234yf where the composition was varied and the vapor pressure was measured at 9.3 and 44.4°C. From the phase study data, the azeotrope composition at other temperatures and pressures was calculated. Table 4 provides the experimental and calculated azeotrope compositions for HF and HFO-1234yf at specified temperatures and pressures.
[0060] Table 4
[0061]
[0062]
Embodiment 3
[0063] Example 3, Experimental Research on the Dew Point and Bubble Point Vapor Pressure of HFO-1234yf
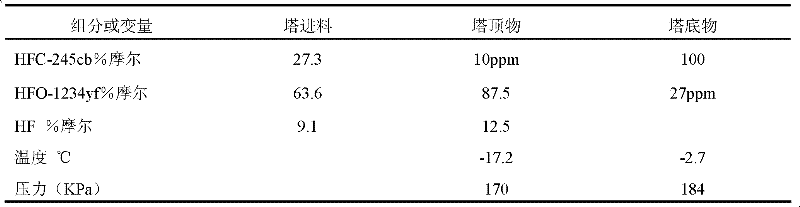
[0064] The dew point and bubble point vapor pressures of the compositions described herein, HFO-1234yf, were calculated from measured and calculated thermodynamic properties, and the near-azeotrope range was determined by such that the difference between the dew point pressure and bubble point pressure (based on the bubble point pressure) was equal to or less than a percent Table 5 summarizes the minimum and maximum concentrations of HFO-1234yf expressed (mole percent, % mole) for the three species.
[0065] table 5
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com