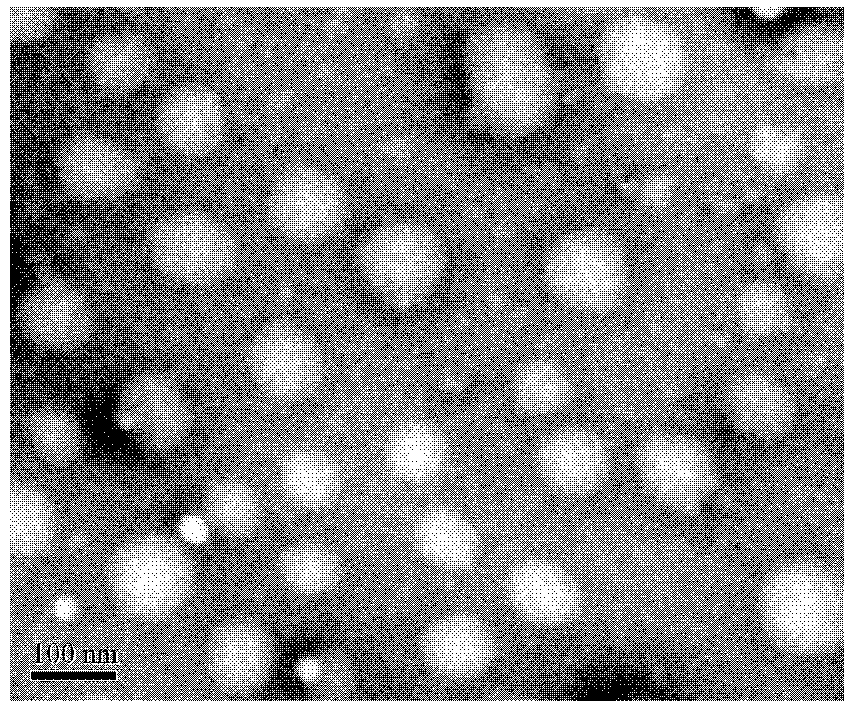
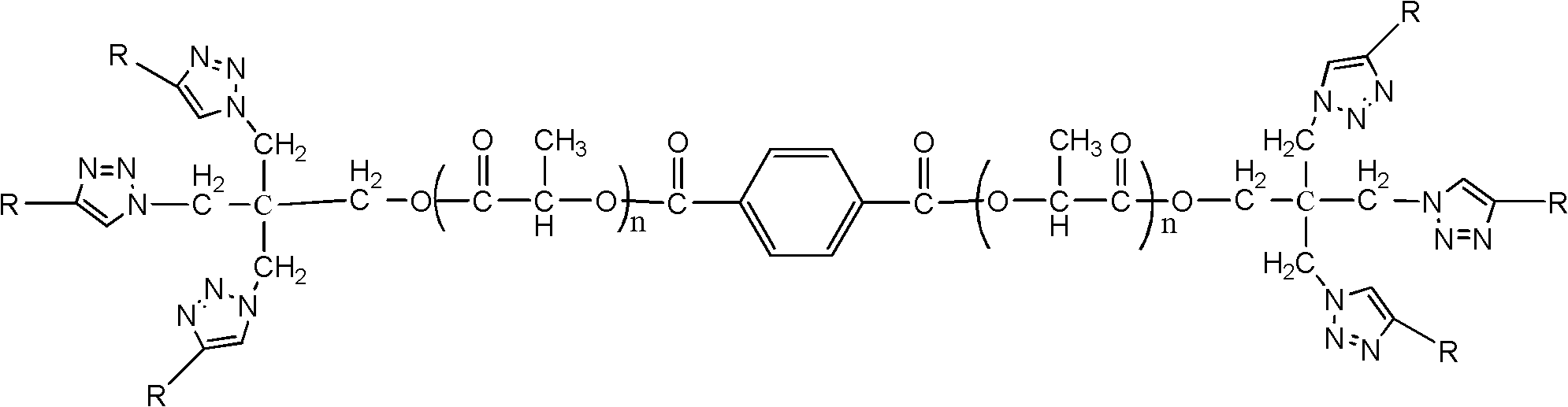
Two-arm hyperbranched star-shaped amphiphilic polylactic acid-poly 2-methacryloyloxyethyl phosphorylcholine block polymer and its preparation method
A technology of methacryloyloxyethylphosphorylcholine and block polymer, applied in two-arm hyperbranched star-shaped amphiphilic polylactic acid-poly2-methacryloyloxyethylphosphorylcholine In the field of alkali block polymers and their preparation, it can solve the problems of less research on hyperbranched star-shaped amphiphilic block polymers, and achieve the effects of prolonging the circulation time in the body, mild conditions, and small critical aggregation concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Click chemistry to synthesize two-arm hyperbranched star-shaped SPLA-b-PMPC polymer: add 0.01g two-arm polylactic acid to the sealed tube, then add 0.05g PMPC containing alkynyl groups, and 0.32,2-bipyridine mg, cuprous bromide 0.1mg and a small stirrer connect the sealing tube to the vacuum line, vacuum and pass nitrogen three times; inject 4ml of anhydrous methanol protected by nitrogen into the sealing tube with a syringe replaced by argon. And 3ml DMF; the material in the sealed tube dissolves quickly, and the solution is reddish brown; put the sealed tube into a thermos cup filled with liquid nitrogen and freeze for 0.5 min, then vacuum for 0.5 min, then pass nitrogen gas, and at the same time warm thaw, repeat the procedure 3 times; seal the tube and freeze for 0.5min, then vacuum for 0.5min, burn the sealed tube with an alcohol blowtorch, finally transfer to an oil bath, react at 0℃ for 2 days; after the reaction is completed, cut the sealed tube with a glass kn...
Embodiment 2
[0047] 1. Click chemistry to synthesize two-arm hyperbranched star-shaped SPLA-b-PMPC polymer: add 500g of two-arm polylactic acid to the sealed tube, then add 100g of PMPC containing alkynyl group, 500mg of 2,2-bipyridine, bromination Connect the sealing tube with cuprous 250mg and a small stirrer to the vacuum line, vacuum and pass nitrogen three times; inject 4ml of anhydrous methanol protected by nitrogen and 3ml DMF into the sealing tube with a syringe replaced with argon; The substance in the tube dissolves quickly, and the solution is reddish brown; put the sealed tube in a thermos cup filled with liquid nitrogen and freeze for 100 minutes, then vacuum for 150 minutes, then pass nitrogen gas, and at the same time warm thaw, repeat this 3 times; seal the tube and freeze again 100min, vacuum for another 150min, burn the sealed tube with an alcohol blowtorch, finally transfer to an oil bath, and react at 200°C for 1 hour; after the reaction is completed, cut the sealed tube ...
Embodiment 3
[0056] 1. Click chemistry method to synthesize two-arm hyperbranched star-shaped SPLA-b-PMPC polymer: add 0.26g of two-arm polylactic acid to the sealed tube, then add 0.6g of PMPC containing alkynyl group and 34mg of 2,2-bipyridine , 19mg cuprous bromide and a small stirrer connect the sealing tube to the vacuum line, vacuum and pass nitrogen three times; inject 4ml of anhydrous methanol protected by nitrogen and 3ml into the sealing tube with a syringe replaced with argon DMF; the substance in the sealed tube dissolves quickly, and the solution is reddish brown; put the sealed tube into a thermos cup containing liquid nitrogen and freeze for 10 minutes, then vacuumize for 15 minutes, then pour nitrogen through it, and at the same time warm thaw, repeat this 3 times; sealing Freeze the tube for another 10 minutes, then vacuum for 15 minutes, burn the sealed tube with an alcohol blowtorch, and finally transfer to an oil bath and react at 35°C for 12 hours; after the reaction is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com