Materials for Organic Electroluminescent Devices
A technology of electronic devices and devices, which is applied in the field of indenofluorene derivatives, can solve problems such as low efficiency, blocking vapor deposition sources, blocking, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
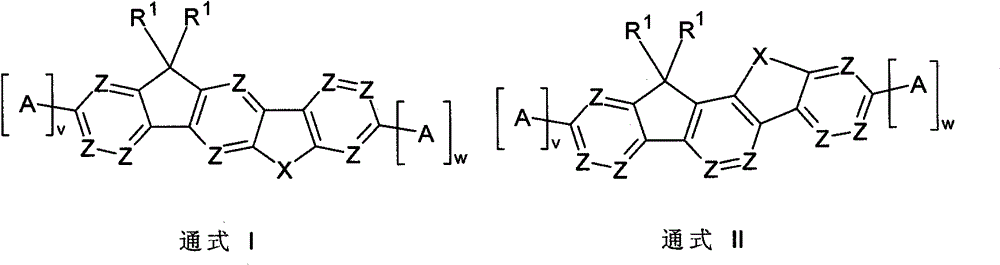
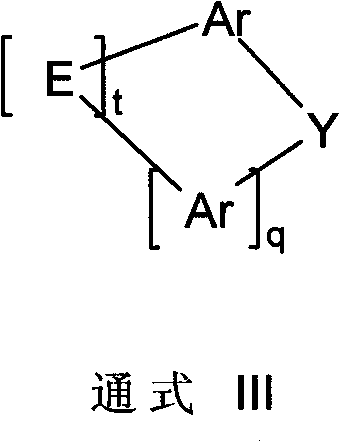
Image
Examples
Embodiment 1
[0103] Example 1: Synthesis of Amine-1
[0104] a) Synthesis of (2'-nitrophenyl) fluoren-2-yl derivatives
[0105]
[0106] Make 164.4g (650mmol) 9,9-dimethyl-9H-fluorene-2-boronic acid, 33.8g (124mmol) 2,5-dibromonitrobenzene and 164.7g (774mmol) K 2 CO 3 Suspended in 750ml THF and 750ml water, the mixture was washed with N 2 Saturation, 2.9 g (2.55 mmol) of tetrakis(triphenylphosphine)palladium(0) were added, and the mixture was heated at the boil for 2 h. The mixture was poured into 3 L of a 1:1:1 mixture of water / MeOH / 6M HCl and filtered with suction to remove a beige precipitate, which was washed with water and dried. according to 1 The product content determined by H-NMR was about 75%, and the total yield was 183 g (90%).
[0107] b) Synthesis of dibromo derivatives
[0108]
[0109] 384 g (973 mmol) of the compound from a) were first introduced under protective gas into 2.5 L of chloroform and cooled to 5°C. To this solution, 55.2ml (1071mmol) of Br dissolv...
Embodiment 2
[0119] Embodiment 2: synthetic amine-2
[0120] a) Synthesis of thioethers
[0121]
[0122] Make the K 2 CO 3 Suspended in 850ml THF and 850ml water, with N 2 The mixture was saturated, 3.3 g (2.9 mmol) of tetrakis(triphenylphosphine)palladium(0) were added and the mixture was heated at the boil for 2 h. The mixture was poured into 3 L of a 1:1:1 mixture of water / MeOH / 6M HCl and filtered with suction to remove a beige precipitate, which was washed with water and dried. according to 1 The product content determined by H-NMR was about 95%, and the total yield was 190 g (82%).
[0123] b) Oxidation of sulfides
[0124]
[0125]196 g (619.3 mmol) of the thioether from a) were first introduced under protective gas into 2.3 L of glacial acetic acid and 250 ml of dichloromethane and cooled to 0°C. To this solution was added dropwise 1.1 L (619 mmol) of 30% H 2 o 2 solution, and the mixture was stirred overnight. Na 2 SO 3 The solution was added to the mixture, the ...
Embodiment 3 to 8
[0141] Examples 3 to 8: Fabrication of OLEDs
[0142] The OLEDs of the invention were produced by the general method according to WO 04 / 058911, which was adapted to the conditions described here (layer-thickness variation, materials used).
[0143] Results for various OLEDs are given in Examples 3 to 8 below. A glass plate coated with structured ITO (indium tin oxide) forms the matrix of the OLED. For improved processing, 20 nm of PEDOT (poly(3,4-ethylenedioxy-2,5-thiophene), applied by spin coating from water, purchased from H.C. Starck, Goslar, Germany) was applied to the substrate superior. The OLED consists of the following sequential layers: substrate / PEDOT 20nm / HIL 15nm / hole transport layer (HTM) 20, 110 or 200nm / NPB 20nm / emissive layer (EML) 30nm / electron transport layer (ETM) 20nm and finally the cathode.
[0144] Materials other than PEDOT were applied by thermal vapor deposition in a vacuum chamber. The emitting layer here always consists of a matrix material (ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com