Preparation method of silicon compound with oxetanyl group
A technology of silicon compounds and organic groups, applied in the field of preparation of silicon compounds with oxetanyl groups, to achieve the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
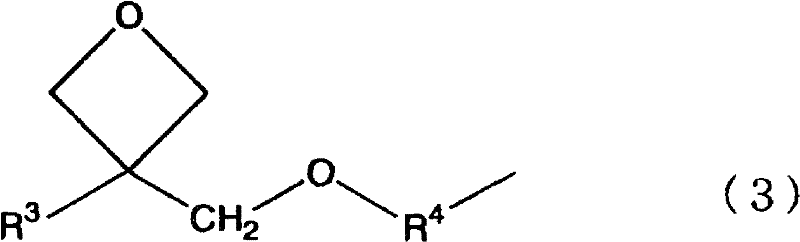
[0030] The preparation method of the silicon compound (C) of the present invention is characterized in that the method includes the following steps: the first step is to make the silicon compound (A) represented by the following general formula (1) and the silicon compound (A) represented by the following general formula (2) The silicon compound (B) shown above is subjected to an alcohol exchange reaction in 1-propanol; in the second step, the composition obtained by performing the above reaction is subjected to hydrolysis and co-condensation under alkaline conditions.
[0031]
[0032] The silicon compound (C) obtained in the present invention is produced as follows: the silicon compound (A), the silicon compound (called AP) produced from the silicon compound (A) through the first step, the silicon compound (B) and the silicon compound through the first process. Step The silicon compound (called BP) produced from the silicon compound (B) is hydrolyzed and co-polycondensed. ...
Embodiment 1
[0113] (Example 1: Preparation of Silicon Compound C1)
[0114] Add 1.1 kg (4.01 mol) of 3-ethyl-3-((3-(trimethoxysilyl) propoxy) represented by the following formula (4) in a reactor equipped with a stirrer and a thermometer Methyl) oxetane (one of the monomers forming the T structural unit in the silicon compound (C) produced, hereinafter referred to as "TMSOX"), 1.1 kg (7.24 mol) tetramethoxysilane (forming A kind of monomer of the Q structural unit, hereinafter referred to as "TMOS") and 1.1kg 1-propanol, then slowly add 0.29kg (6.8mol methanol, 0.8mol tetramethylammonium hydroxide) 25% tetramethylhydroxide ammonium solution in methanol. The reaction was carried out at 60° C. for 1 hour, and then, while stirring the reaction solution, a mixed solution of 750 g (41 mol) of water and 750 g of 1-propanol was added dropwise over 0.5 hours. After reacting at 60 degreeC for 6 hours including the time of dropping, nitric acid was added and neutralized to the reaction liquid. T...
Embodiment 2
[0122] (Example 2: Preparation of Silicon Compound C2)
[0123] Add 27.82g (0.1mol) TMOSOX, 28.19g (0.185mol) TMOS and 111.5g 1-propanol shown in formula (4) in the reactor that is provided with stirrer and thermometer, then slowly add 7.29g (0.17mol) Methanol, 20mmol tetramethylammonium hydroxide) 25% tetramethylammonium hydroxide methanol solution. After reacting at 60° C. for 1 hour, a mixed solution of 18.72 g (1.04 mol) of water and 20.0 g of 1-propanol was added dropwise over 0.5 hours. After reacting at 60 degreeC for 6 hours including the dripping time, nitric acid was added and neutralized. The organic solvent and water were distilled off under reduced pressure, and the obtained residue was dissolved in PGMEA and washed with water to remove salts and excess acid. The solvent was distilled off from the obtained PGMEA solution under reduced pressure to obtain a colorless solid (compound C2). Yield was 28.8 g. The mass yield is 90%.
[0124] For silicon compound C2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com