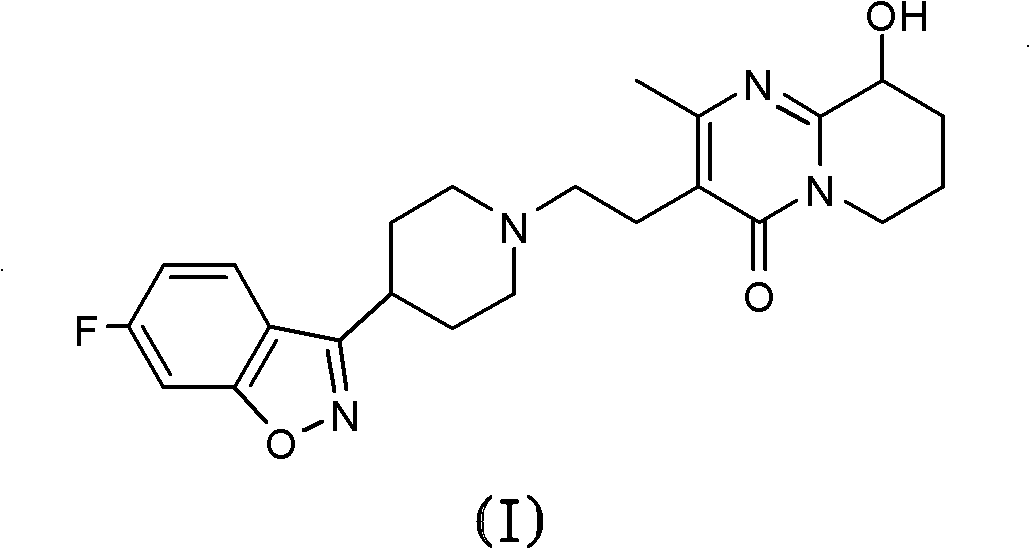
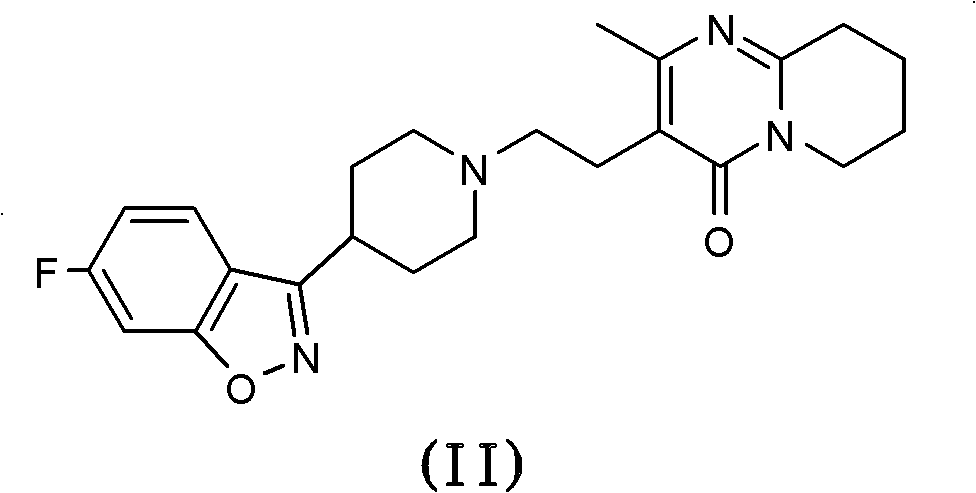
One-step process for the preparation of paliperidone and its oxalate
A kind of technology of paliperidone and trialkyl phosphite, applied in the one-step field of preparing paliperidone and its oxalate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of LiHMDS
[0056] Place a small amount of 2,2'-bipyridine crystals in a 25mL round bottom flask, and place the system under an argon atmosphere. Anhydrous toluene (2 mL) was added, the solution was cooled to -10°C, freshly distilled hexamethyldisilazane (735 μL, 3.52 mmol) was added, followed by n-butyllithium (1.6M n-hexane solution, 2.0 mL, 3.2 mmol). The mixture was stirred at -10°C for 30 minutes to obtain an approximately 0.68M LiHMDS red solution.
Embodiment 2
[0058] oxidation reaction
[0059] Connect the 50 mL two-neck round bottom flask to a compressed air pump (GC grade, H 2 O2 SO 4 Dry and remove solvent under reduced pressure. The residue was purified by flash chromatography on silica gel (20 g) eluting with a gradient of dichloromethane / methanol (90:10 to 86:14) to give the ketone by-product (43 mg, 8%) as a yellow oil in the initial fraction, Paliperidone (342 mg, 66%) was subsequently obtained as a white foam. The product was further purified by crystallization from ethyl acetate / n-hexane to obtain a product with a melting point of 162.2-162.4°C.
[0060] 1 H NMR (300MHz, CDCl 3 , 298K) δ1.77 (1H, m center); 1.89-2.04 (1H, m); 2.07-2.21 (6H, m); 2.25-2.42 (2H, m); 2.36 (3H, s); 2.55 and 2.79 (4H, A and X are part of an AA'XX' system); 3.10 (1H, m center); 3.18 (2H, width d, J 11.4Hz); 3.87-4-03 (2H, m); 4. .12 (1H, wide s); 4.51 (1H, dd, J6.3 and 10.2Hz); 7.07 (1H, dt, J2.1 and 15.3Hz); 7.25 (1H, dd, J2.4 and 9.0Hz...
Embodiment 3
[0063] Purification of paliperidone by salt formation
[0064] The crude product paliperidone (theoretical yield 24.4 mmol) obtained from the oxidation reaction of 10 g risperidone was dissolved in methanol (100 mL) after the above-mentioned aqueous treatment, and oxalic acid (1.3 g, 14.4 mmol).
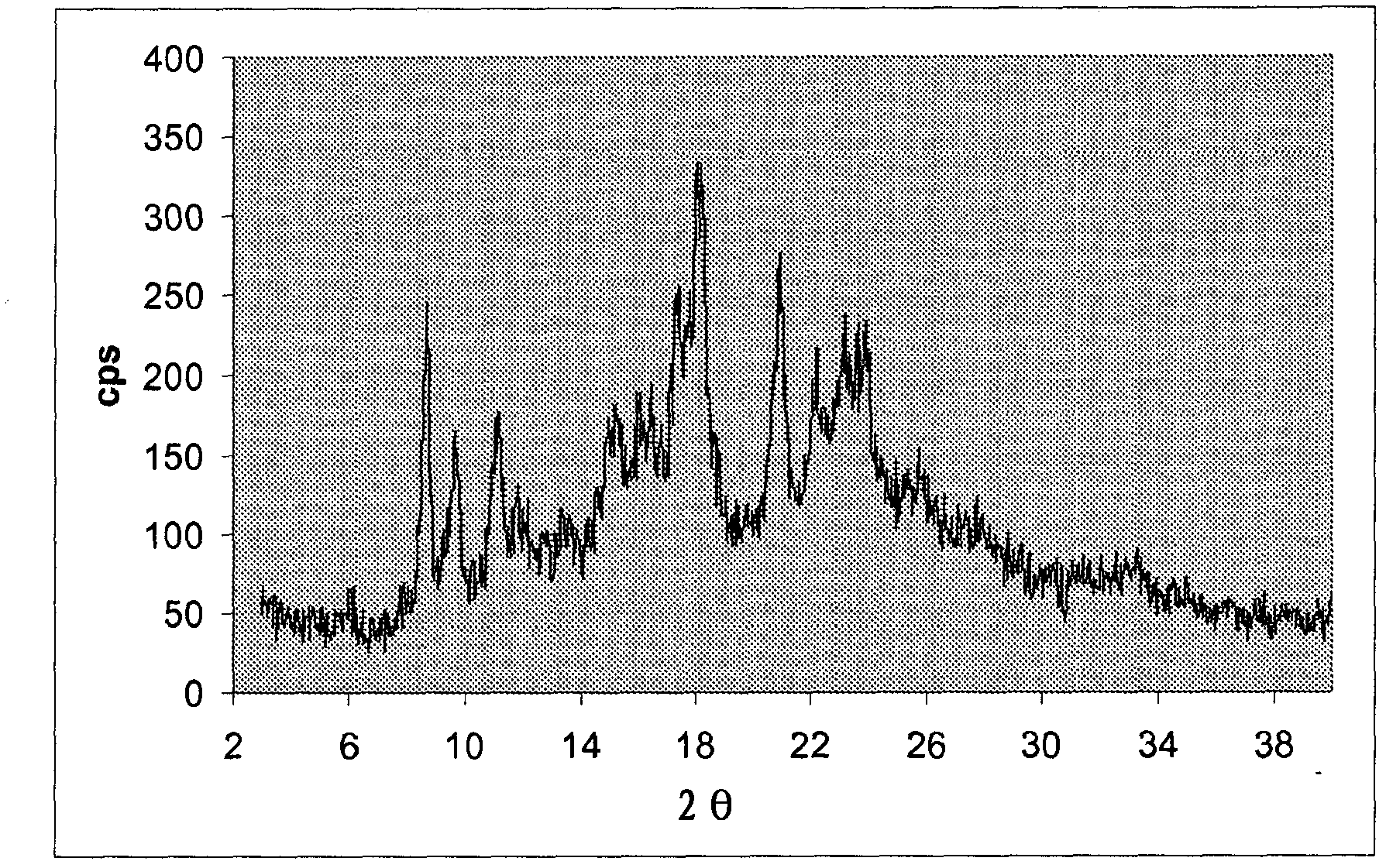
[0065] The crystalline material was filtered and washed with methanol to obtain paliperidone oxalate (7.2 g, 60% yield) as a colorless solid, mp 135-140° C., shown as figure 1 XRPD pattern.
[0066] Paliperidone was obtained quantitatively from the oxalate salt (7.2 g, 13.9 mmol), treated with 20% ammonium hydroxide, extracted with dichloromethane (50 mL), washed with brine, dried the organic extract over sodium sulfate, and concentrated to solid residue. Paliperidone can optionally be recrystallized from acetonitrile.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com