A kind of preparation method of high-purity capecitabine
A capecitabine, high-purity technology, applied in the field of medicine and chemical industry, can solve the problems of poor impurity removal effect, cumbersome operation, and high cost, and achieve the effect of good removal effect, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
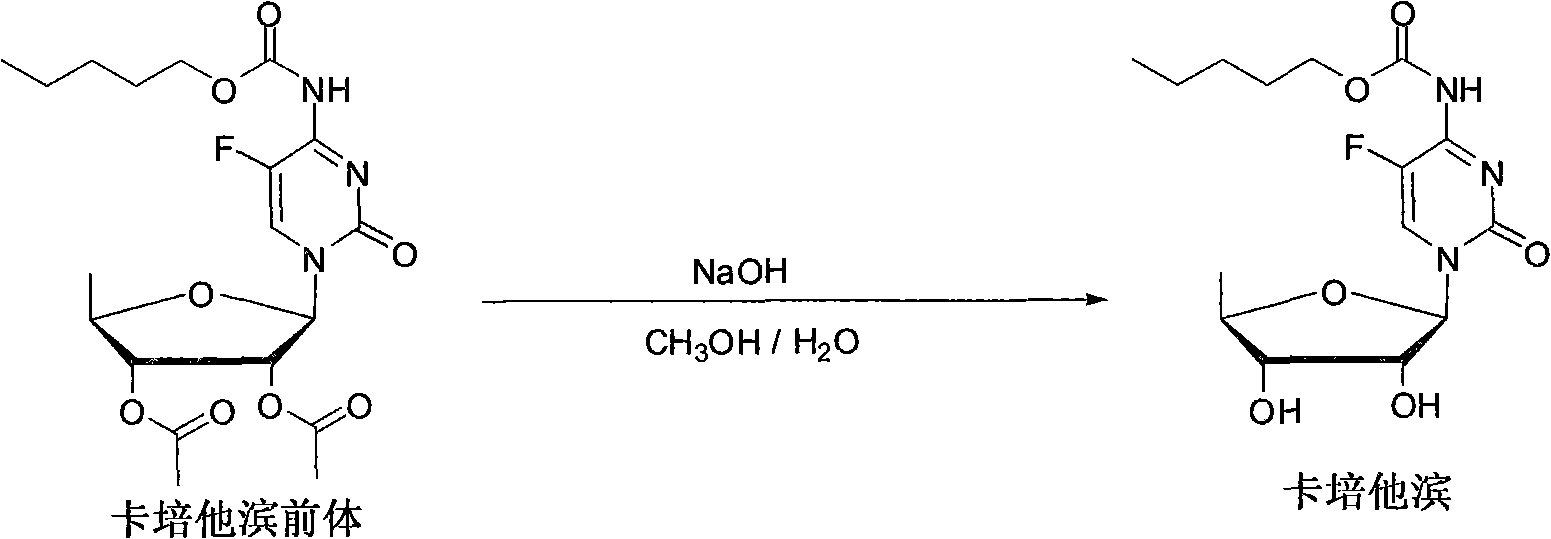
[0034] Add capecitabine precursor-5′-deoxy-2′,3′-di-O-acetyl-N-[(n-pentyloxy)carbonyl]-5-fluorocytidine 16g into a 250ml three-necked flask, Add 100ml of methanol to dissolve, cool down to 0°C, add 27ml of 2N sodium hydroxide solution dropwise, keep the temperature for 3-5 hours, add 2N hydrochloric acid dropwise to adjust the pH to 6-7, evaporate most of the methanol under reduced pressure, and add 40ml of methyl The organic layer was extracted with tert-butyl ether, the aqueous layer was extracted twice with 30ml methyl tert-butyl ether, the organic layers were combined, washed with 50ml of saturated saline, washed with 25ml of water, dried over anhydrous sodium sulfate, filtered, and added dropwise at 25°C Heptane 500ml, precipitated product, filtered, and vacuum dried at 40°C for 6 hours to obtain capecitabine as a white solid, dry weight: 11.6g, yield: 93.2%, HPLC purity: 99.83%. m.p.112.5~113.5℃; 1 H-NMR (400Hz, DMSO-d 6 )δ: 0.90-0.93 (t, J=6.9Hz, 3H, CH 2 CH 3 ), 1....
Embodiment 2
[0036]Add capecitabine precursor-5'-deoxy-2',3'-di-O-acetyl-N-[(n-pentyloxy)carbonyl]-5-fluorocytidine 20g into a 250ml three-necked flask, Add 100ml of methanol to dissolve, cool down to 0°C, add 34ml of 2N sodium hydroxide solution dropwise, keep the temperature for 3 hours, add 2N hydrochloric acid dropwise to adjust the pH to 6-7, evaporate most of the methanol under reduced pressure, add 40ml of dichloromethane to extract The organic layer and the aqueous layer were extracted twice with 25ml of dichloromethane, the organic layers were combined, washed with 60ml of saturated brine, washed with 60ml of water, dried over anhydrous sodium sulfate, filtered, and 290ml of n-hexane was added dropwise at 25°C to precipitate the product, filtered, Vacuum drying at 40°C for 6 hours yielded capecitabine as a white solid, dry weight: 14.5 g, yield: 89.5%, HPLC purity: 99.86%.
Embodiment 3
[0038] Add 50 g of capecitabine precursor—5′-deoxy-2′,3′-di-O-acetyl-N-[(n-pentyloxy)carbonyl]-5-fluorocytidine into a 1L three-necked flask, Dissolve in 500ml of methanol, cool down to -5°C, add 85ml of 2N sodium hydroxide solution dropwise, keep the temperature for 5 hours, add 2N hydrochloric acid dropwise to adjust the pH to 6-7, evaporate most of the methanol under reduced pressure, add 100ml of methyl tert-butyl Extract the organic layer with base ether, extract the aqueous layer twice with 60ml methyl tert-butyl ether, combine the organic layers, wash with 150ml of saturated brine, wash with 100ml of water, dry over anhydrous sodium sulfate, filter, and add 720ml of n-hexane dropwise at 25°C , the product was precipitated, filtered, and vacuum-dried at 40° C. for 6 hours to obtain a white solid, namely capecitabine, with a dry weight of 36.8 g, a yield of 91%, and a HPLC purity of 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com