Certain crystalline hydrates,pharmaceutical compositions thereof and methods for preparation and use thereof
A technology of crystalline hydrates and hydrates, which is applied in the direction of drug combinations, pharmaceutical formulations, antibacterial drugs, etc., and can solve problems such as unpredictable properties and no discovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] In some embodiments, (S)-[N-3-(3'-fluoro-4'-(4"-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl] The preparation method of methyl acetamide 1 / 2 crystalline hydrate comprises:
[0094] - (S)-[N-3-(3'-fluoro-4'-(4"-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylacetamide Dissolved in an acidic solvent with a pH value≤5 or in a mixed solvent comprising at least one non-acidic organic solvent and at least one acidic solvent with a pH value≤5 to form (S)-[N-3-(3'-fluoro-4 '-(4"-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylacetamide solution;
[0095] Wherein at least one acidic solvent and at least one non-acidic organic solvent are mixed in an appropriate volume ratio, such as 1:9 to 9:1, further such as 2:8 to 8:2 or 3:7 to 7:3 by volume than mixed;
[0096] - Stir the solution at an appropriate temperature, such as 35°C to 80°C, further such as 35°C to 70°C, further such as 35°C to 60°C, for a certain period of time, such as stirring for at least 1 hour, s...
example 1
[0174] Example 1: (S)-[N-3-(3′-fluoro-4′-(4″-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylethyl Preparation of amides (crude)
[0175] (S)-[N-3-(3′-fluoro-4′-(4″-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylacetamide (crude ) can generally be synthesized by the method disclosed in CN1355165A.
[0176] (1) Preparation of 3-fluoro-4-(4'-phenylpiperazinyl)nitrobenzene
[0177] Add 50ml of ethyl acetate, 13.5ml of 4-phenylpiperazine and 15.30ml of diisopropylethylamine into a 250ml three-necked flask. With magnetic stirring at room temperature, 9.0 ml of 3,4-difluoronitrobenzene was added. After reacting for 105 hours, the reaction solution was poured into water, extracted 3 times with ethyl acetate (150ml×3), the extract was washed 3 times with saturated sodium chloride solution (150ml×3), and anhydrous magnesium sulfate (MgSO 4 ) was dried and evaporated to dryness. An orange-yellow solid was obtained. Acetone:water (volume ratio 9:1) was recrystallized to ...
example 2
[0195] Example 2: (S)-[N-3-(3′-fluoro-4′-(4″-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylethyl Preparation of Amide 1 / 2 Hydrate
[0196] (S)-[N-3-(3'-fluoro-4'-(4"-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidine prepared by the method described in Example 1 Base] methyl acetamide crude product 1g joins in the 6wt% hydrochloric acid of 2ml.Add a small amount of gac, heat and stir at 50 ℃ for 2 hours, filter.Filtrate is placed crystallization at room temperature, and gained crystal is vacuum-dried at 40 ℃ for 5 hours. Content measured by HPLC: 99.0%.Melting point: 210-215°C (capillary measurement).
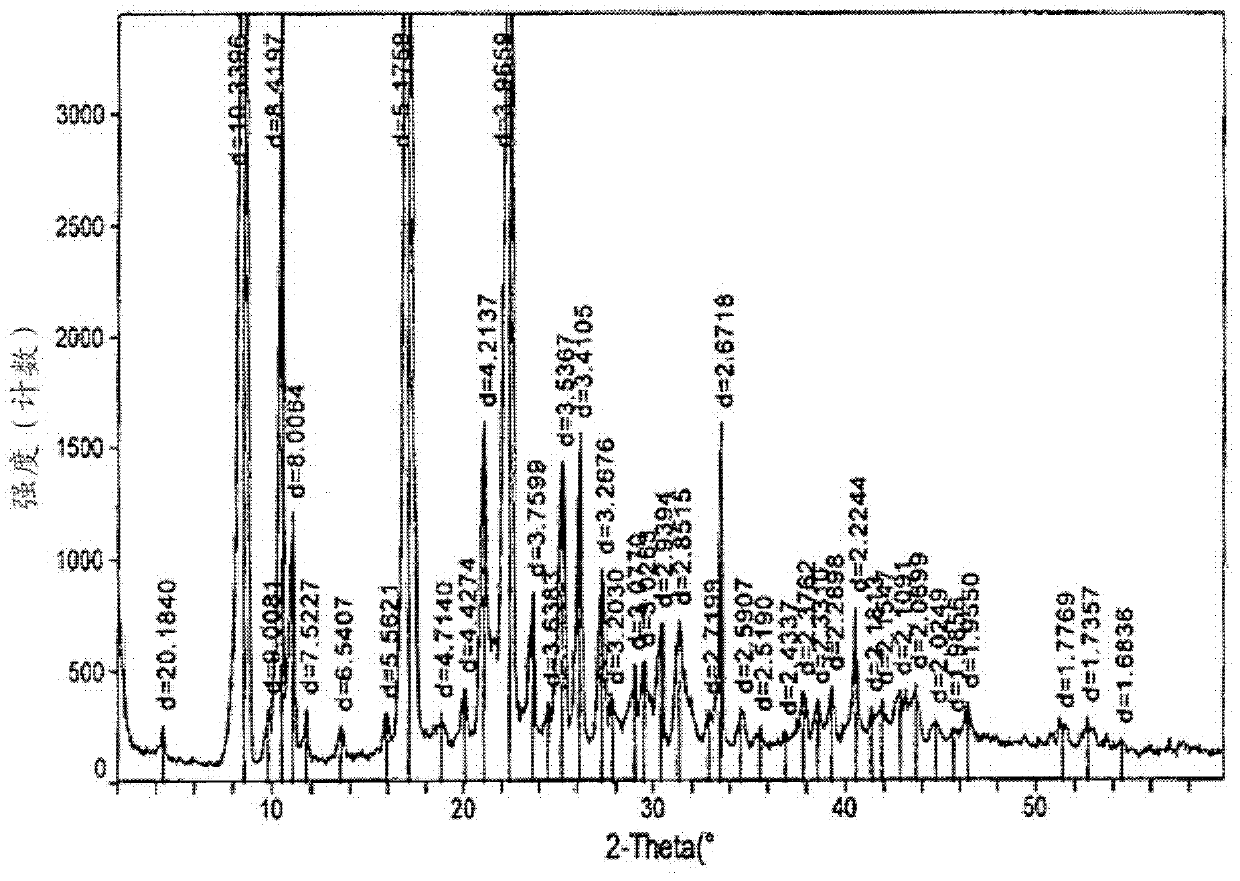
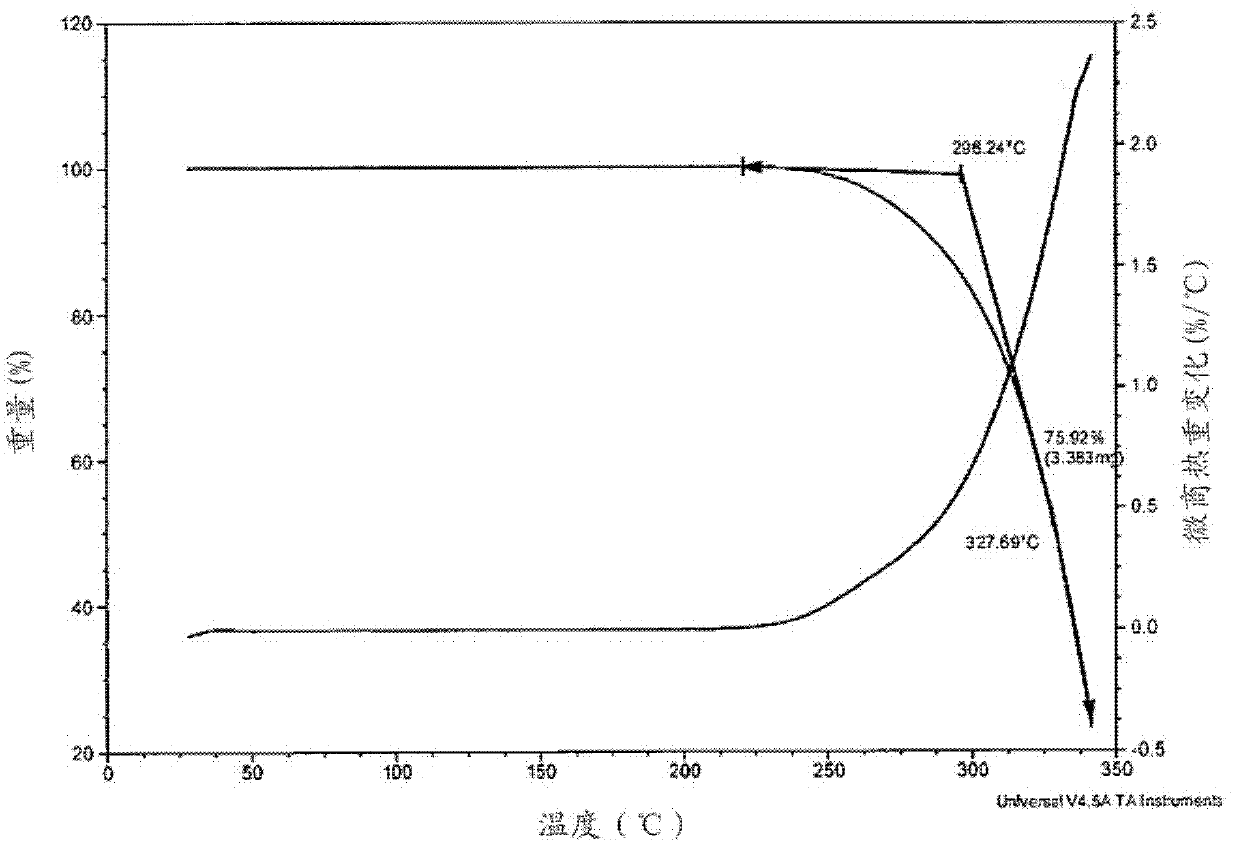
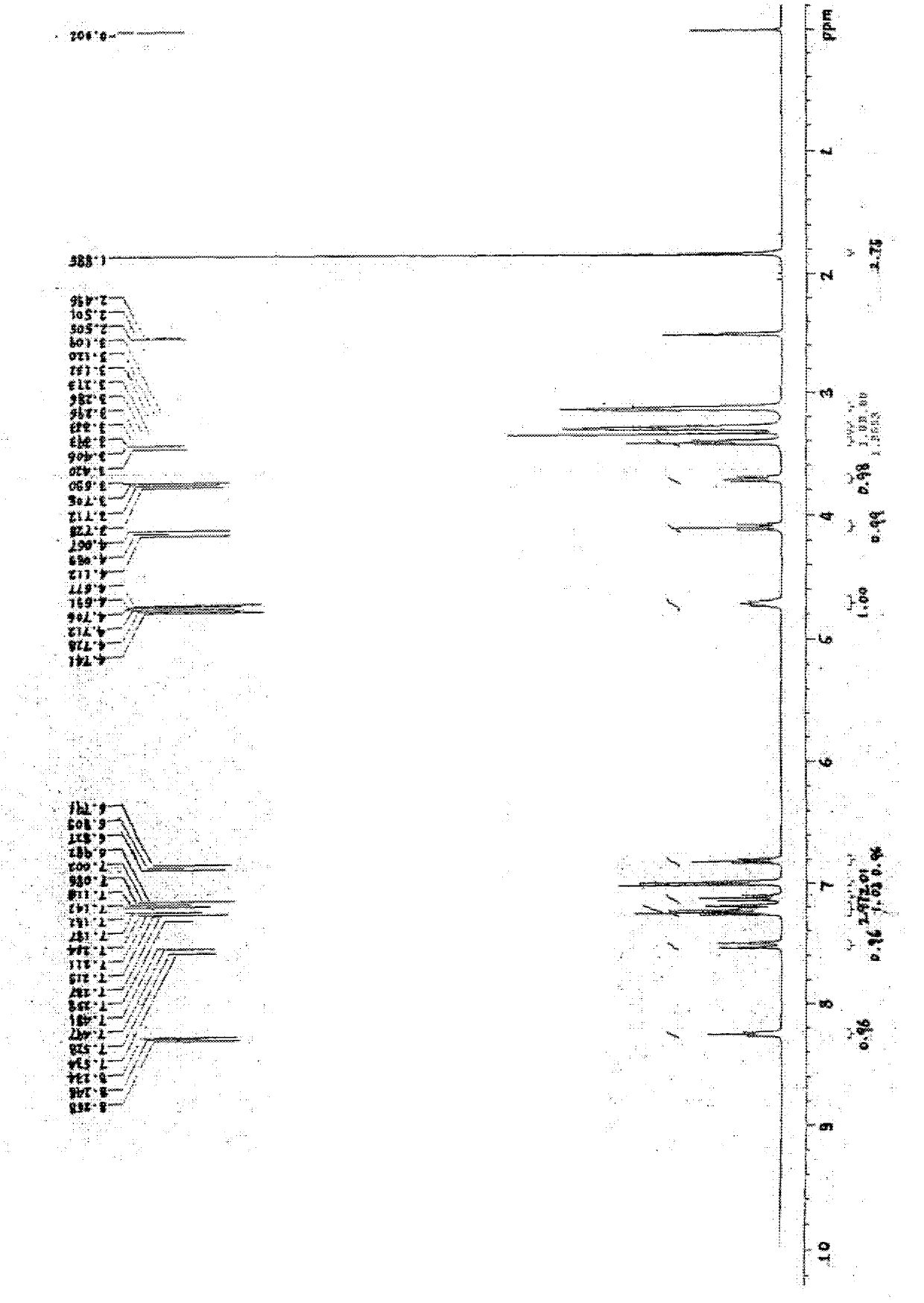
[0197] Figure 7-13 For the obtained (S)-[N-3-(3'-fluoro-4'-(4"-phenylpiperazinyl))phenyl-2-oxo-5-oxazolidinyl]methylacetamide Hydrate X-ray diffraction pattern, TGA, 1 H-NMR, 13 C-NMR, IR and DSC thermal analysis spectra.
[0198] X-ray powder diffraction measurement conditions are: CuKa rays, 1.54 monochromator, tube voltage 40KV, tube current 25mA. In the X-ray powd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com