40H-GTS-21 compounds, preparation method and application thereof
A 4OH-GTS-21, 1.4OH-GTS-21 technology, applied in the field of compound drugs, can solve the problems of limited application and low blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
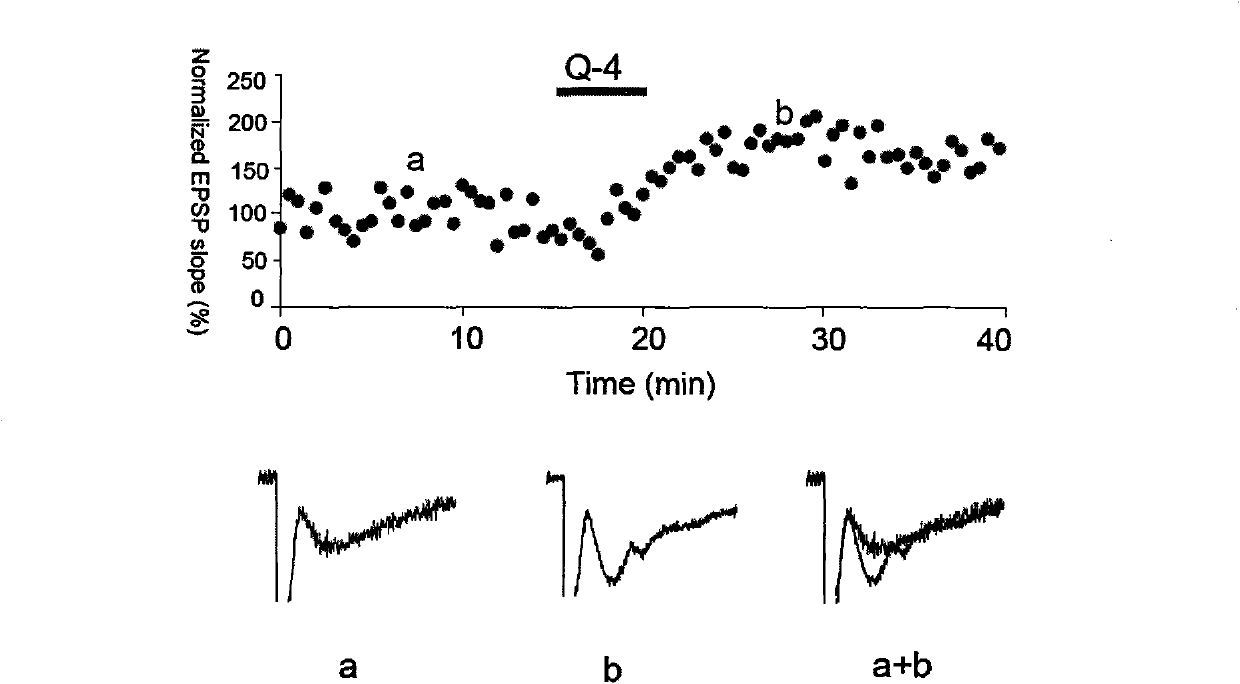
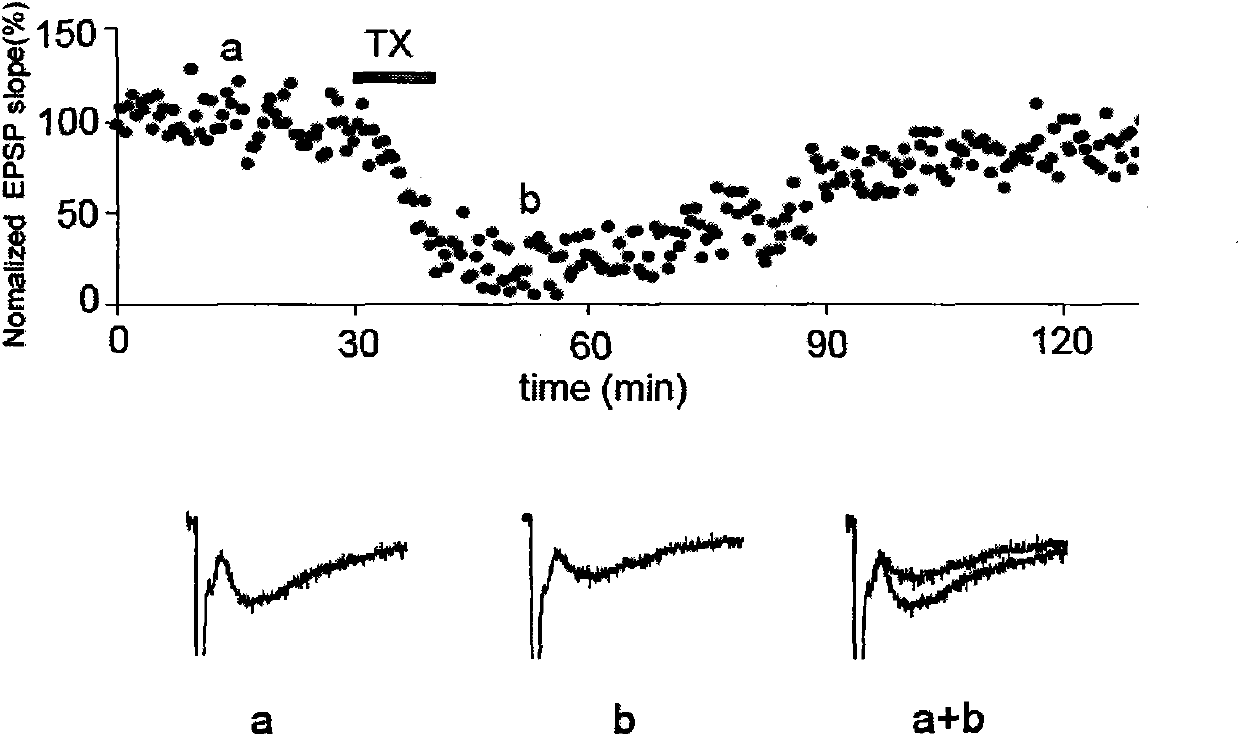
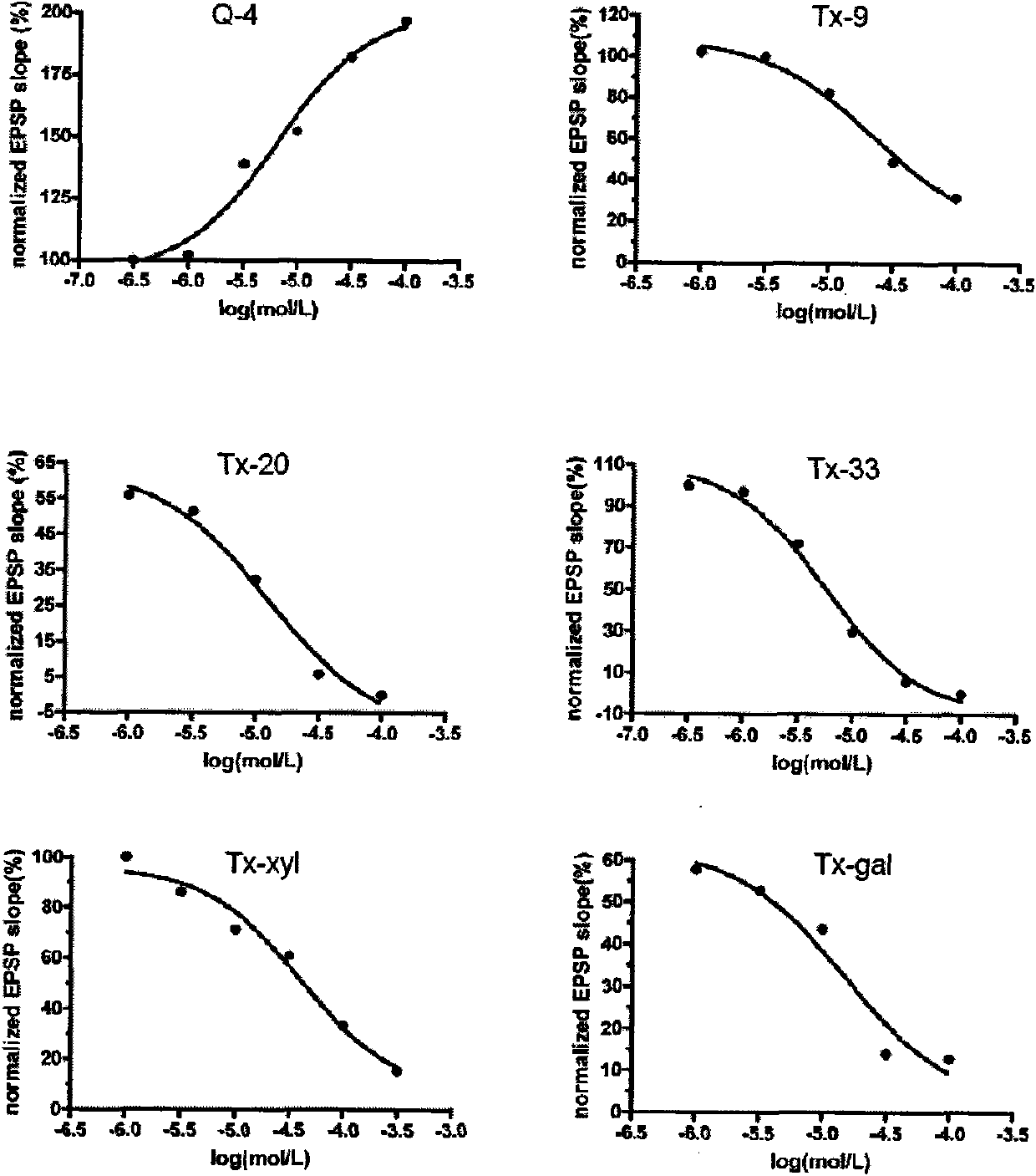
[0032] Example 1, preparing the 4OH-GTS-21 compound shown in the general formula, where R1 = difluoromethoxy, R3 = H, and X = O, the compound is named Q4. The preparation method is as follows:
[0033]
[0034] Synthesis of Intermediate 2:
[0035] In 500mL eggplant-shaped bottle, add 2,4-dihydroxybenzaldehyde (10g, 72.4mmol), K 2 CO 3 (12g, 86.9mmol), acetone (200mL) and a catalytic amount of tetrabutylammonium iodide (2.6g, 7.2mmol), added benzyl bromide (8.64mL, 72.4mmol) at room temperature, heated to reflux for 2h, followed by TLC process, remove potassium carbonate by filtration after the reaction, concentrate under reduced pressure, dilute the residue with dichloromethane (600ml), wash with water (100ml×2), combine the organic phases and dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. Compound 2 (13.4g, 64.8%) was obtained by analysis. 1 H NMR (CDCl 3 , 600MHz) δ=11.48(s, 1H, OH), 9.72(s, 1H, CHO, 7.45-7.41(m, 5H, ...
Embodiment 2
[0043] Example 2, preparation of the following general formula 4OH-GTS-21 compound, wherein R1 = methoxy, X = O, R3 includes 1,2-trans-D-glucopyranosyl, 1,2-trans- D-xylopyranosyl, 1,2-trans-D-galactopyranosyl:
[0044]
[0045] Synthesis of intermediates 12-14:
[0046] Dissolve 0.41 mmol of compound 9 (or 10, or 11) and compound 8 (100 mg, 0.34 mmol) in CHCl 3 (10mL) and H 2 In the mixed solvent of O (10mL), add K 2 CO 3 (117.3mg, 0.85mmol) and tetrabutylammonium bromide (10.9mg, 0.03mmol) were stirred at 50°C for 5h. TLC detects the reaction progress, after the reaction finishes, add cold water (10mL) to dilute, with CHCl 3 (3×50mL) extraction, the combined organic phases were washed successively with 5% NaOH (2×50mL) and saturated brine (2×50mL), anhydrous NaOH 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure and subjected to silica gel column chromatography (chloroform:methanol=80:1) to obtain the corresponding compound 12 ...
Embodiment 3
[0055] Example 3, preparation of 4OH-GTS-21 compounds represented by the general formula, wherein R1 = methoxy, R3 = 1,2-trans-D-glucopyranosyl, X includes OCH 2 CO(CH 2 ) 3 , OCH 2 CO(CH 2 ) 2 When O:
[0056]
[0057] Synthesis of intermediate 16
[0058] Compound 15 (5.0 g, 12.8 mmol) was weighed and dissolved in 40 mL of tetrahydrofuran, 2 mL of benzylamine was added and stirred at room temperature. After the reaction was detected by TLC, it was concentrated under reduced pressure. The resulting residue was subjected to silica gel column chromatography, eluting with ethyl acetate:petroleum ether=1:4, to obtain 3.14 g of red liquid, with a yield of 64%.
[0059] Synthesis of Intermediate 17
[0060] Dissolve 16 (2.2 g, 5.7 mmol) in 30 mL of dry CH 2 Cl 20.43mL 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) was added dropwise under ice-cooling, then 4.6mL trichloroacetonitrile was slowly added, and stirred at room temperature for 2h. The end of the reaction was dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com