Method for synthesizing adefovir serving as anti-hepatitis B virus medicine
A synthetic method and anti-hepatitis B technology, applied in the fields of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of low synthesis process yield, many side reactions, unsuitable for industrial production, etc. , to achieve good selectivity, improved reaction selectivity, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
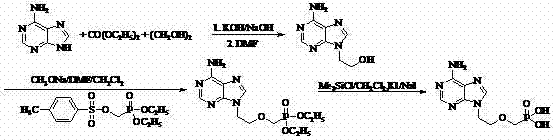
[0041] Synthesis of 9-(2-hydroxyethyl)adenine:
[0042] At room temperature and under nitrogen protection, 27 g (0.200 mol) of adenine, 100 mL of DMF, and 0.3 g of sodium hydroxide were put into a 250 mL reaction flask. Then ethylene oxide gas was passed through the system, and detected by TLC and HPLC until the adenine disappeared. DMF was distilled off under reduced pressure, 80 mL of absolute ethanol was added, refluxed for 2 h, and filtered to obtain 33.3 g of 9-(2-hydroxyethyl)adenine as a white solid with a yield of 94% and a purity of more than 99% by HPLC.
[0043] Synthesis of 9-(2-(diethylphosphonomethoxy)ethyl)adenine:
[0044] At 80°C, add 10.0 g (0.056 mol) of 9-(2-hydroxyethyl)adenine into 50 mL of DMF, then add 4.8 g (0.028 mol) of magnesium tert-butoxide, and react for 0.5-1 h. Add 18.0 g (0.056 mol) of diethyl p-toluenesulfonyloxymethylphosphonite, react for 7~8 h, add acetic acid to neutralize the excess alkali to neutral, evaporate DMF, and use ethyl aceta...
Embodiment 2
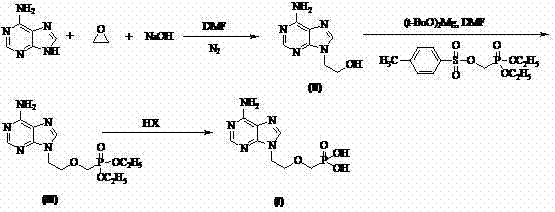
[0048] Synthesis of 9-(2-hydroxyethyl)adenine:
[0049] At room temperature and under the protection of nitrogen, 54.0 g (0.400 mol) of adenine, 150 mL of DMF, and 0.6 g of sodium hydroxide were added at one time. Then ethylene oxide gas was passed through the system, and detected by TLC and HPLC until the adenine disappeared. DMF was distilled off under reduced pressure, and recrystallized using ethanol-water system (V:V=2:8) to obtain 62.3 g of white solid 9-(2-hydroxyethyl)adenine, with a yield of 87% and a purity of more than 99% by HPLC.
[0050] Synthesis of 9-(2-(diethoxyphosphonomethoxy)ethyl)adenine:
[0051] At 80°C, add 17.9 g (0.100 mol) of 9-(2-hydroxyethyl) adenine into 50 mL of DMF, then add 8.5 g (0.050 mol) of magnesium tert-butoxide, and react for 0.5-1 h , add 32.2 g (0.100 mol) of diethyl p-toluenesulfonyloxymethylphosphonite, react for 7~8 h, add p-toluenesulfonic acid to neutralize excess alkali to neutral, distill DMF, and use ethyl acetate ( 300 mL ×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com