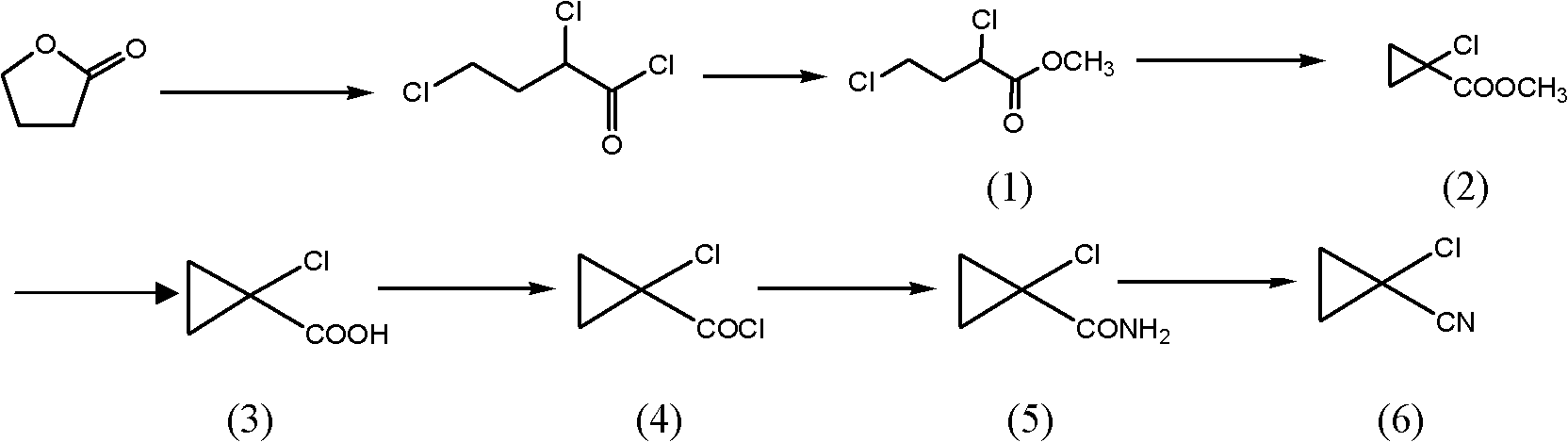
Preparation method of cyclopropyl fenpropathin derivative
A technology of bromocyclopropylmethyl cyanide and derivatives, which is applied in the field of preparation of cyclopropylmethyl cyanide derivatives, can solve problems such as unfavorable industrial production, influence on reaction yield, cumbersome operation, etc., achieve method safety and increase reaction temperature , the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
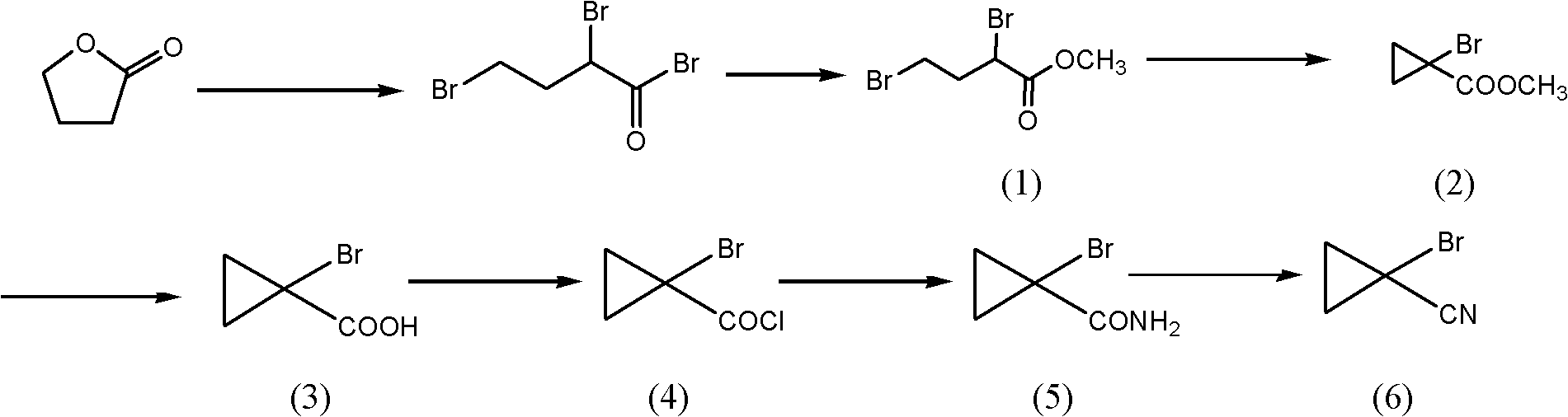
[0043] Embodiment 1 prepares 1-bromocyclopropyl methyl cyanide
[0044] The process route is as follows:
[0045]
[0046] (1) Synthesis of methyl 2.4-dibromobutyrate
[0047] Put 17kg of γ-butyrolactone and 21.6kg of phosphorus tribromide into the glass-lined reactor, lower the temperature to -10°C under the protection of nitrogen, slowly add 58kg of bromine, and absorb the tail gas with a three-stage absorption tower, and control the reaction temperature to 40 ~50°C; heat up for 1 hour after adding, then raise the temperature to 80°C and hold for 3 hours. After the reaction, the temperature was lowered to -5°C, 14.2 kg of methanol was added dropwise, and the reaction was kept at -5 to 5°C for 1 hour after the addition was completed.
[0048] Add 100kg methyl tert-butyl ether, 15kg tap water in the above-mentioned product, use 10%Na 2 CO 3 The pH of the solution was adjusted to 7, the layers were allowed to stand, and the oil layer was washed once with 10 kg of tap wat...
Embodiment 2
[0062] Embodiment 2 prepares 1-bromocyclopropyl methyl cyanide
[0063] (1) Synthesis of methyl 2.4-dibromobutyrate
[0064] Put 86g of γ-butyrolactone and 11.8g of red phosphorus into the four-necked reaction bottle, lower the temperature to -10°C under the protection of nitrogen, slowly add 665g of bromine dropwise, absorb the tail gas with liquid alkali, and keep the temperature in the bottle at 40-50°C ; Add and keep warm for 1 hour, heat up to 80°C and keep warm for 3 hours. Cool down to -2°C, add 80 g of methanol dropwise, and keep the reaction at -5 to 5°C for 1 hour after the addition.
[0065] Add 400g of chloroform, 70g of tap water to the above product, and then use 10%Na 2 CO 3 The pH value of the aqueous solution was adjusted to 7, and the layers were allowed to stand. The oil layer was washed once with 70 g of tap water, and all the water layers were treated as wastewater. The oil layer was distilled to remove the solvent to obtain 2.4-dibromobutyric acid meth...
Embodiment 3
[0076] Embodiment 3 prepares 1-bromocyclopropyl methyl cyanide
[0077] The preparation method is the same as in Example 2, but step (3) synthesizes 1-bromocyclopropyl carboxylic acid and proceeds as follows: in a four-necked glass reaction flask, add 120 g of methanol, 50 g of 1-bromocyclopropyl formate methyl ester, 82% Potassium hydroxide 17g, react at 0-10°C for 15 hours, distill off the solvent, add 100g tap water to the remaining solid, adjust the pH to 1-2 with concentrated hydrochloric acid, add methyl tert-butyl ether for extraction 3 times, combine the oil layers with After washing with tap water once, the oil layer was distilled off under reduced pressure to remove the solvent to obtain 41.2 g of solid 1-bromocyclopropylcarboxylic acid with a yield of 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com