Nitro imidazole compound, its preparation method and application
A compound and hydrate technology, applied in the fields of pharmacy, medicinal chemistry and pharmacology, can solve the problems of low water solubility, low bioavailability and poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
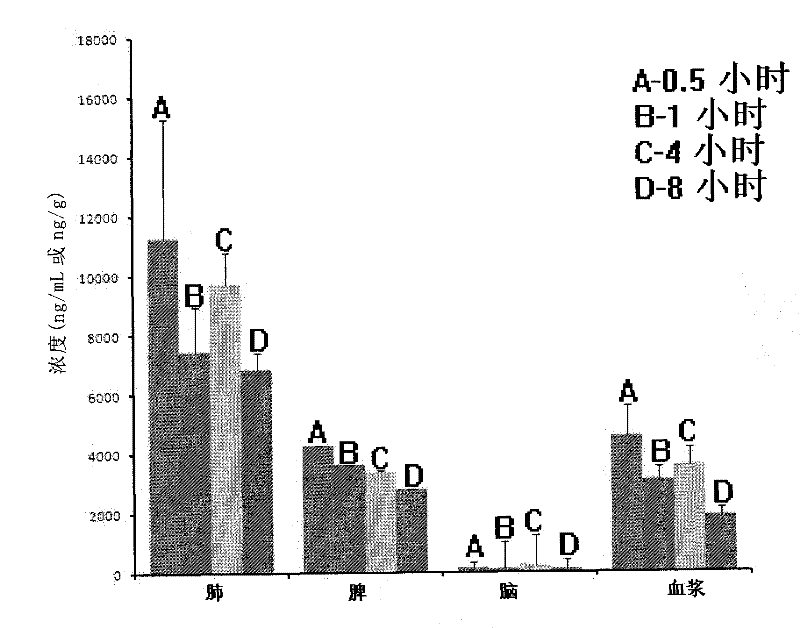
Image
Examples
preparation example Construction
[0059] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent (usually a polar aprotic solvent) at -30°C to solvent reflux temperature (preferably -20 to 80°C). The reaction time is usually 0.1 to 60 hours, preferably 0.5 to 48 hours.
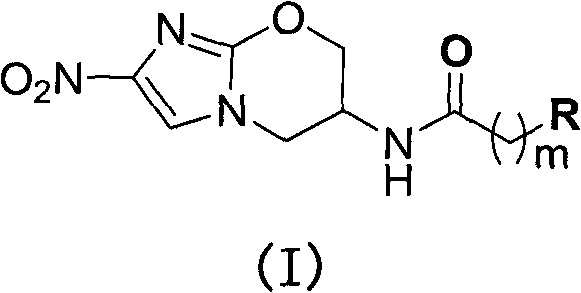
[0060] Describe the preparation of structural formula (I) compound more specifically below:
[0061] Process 1
[0062]
[0063] (1) Intermediate I-1 is subjected to Mitsunobu reaction with various substituted phenols in the presence of triphenylphosphine and azo active esters to obtain intermediates. The Boc protecting group is removed under acidic conditions to obtain intermediates I-6a-I -6f. The solvent can be selected from tetrahydrofuran, 1,4-dioxane, methyl tert-butyl ether, etc.; the azo active ester can be selected from DEAD, DIAD, etc. The optimal reaction condition is, in the presence of triphenylphosphine and DIAD, using tetrahydrofuran as a solvent, react at -10°C to room ...
specific Embodiment approach
[0105] The present invention is explained more specifically in the following examples. It should be understood, however, that these examples are given to illustrate the invention and not to limit the scope of the invention in any way. For the experimental methods without specific conditions indicated in the following examples, the conventional conditions or the conditions suggested by the manufacturer are usually followed. Parts and percentages are by weight unless otherwise indicated.
[0106] In all examples, the melting point is determined with an X-4 melting point apparatus, and the thermometer is not corrected; 1 H NMR was recorded with a VarianMercury 400 or 600 nuclear magnetic resonance instrument, and the chemical shift was expressed in δ (ppm); MS was measured with a Shimadzu LC-MS-2020 mass spectrometer. The silica gel used for separation is not specified and is 200-300 mesh, and the ratio of the eluent is the volume ratio.
preparation example 1
[0108] 4-(4-Trifluoromethoxy)phenoxy)piperidine (I-6a)
[0109] p-Trifluoromethoxyphenol (32.7g, 184mmol), N-Boc-4-hydroxypiperidine (37g, 184mmol), triphenylphosphine (48.3g, 184mmol) were dissolved in dry THF (500mL), ice DIAD (37.2 g, 184 mmol) was added dropwise under cooling in a bath, and stirred overnight at room temperature after the drop was completed. The THF was spinned off, the residue was extracted with petroleum ether, and the extract was concentrated to obtain 71.2 g of a light yellow oil. The yield of the crude product was over 100%, and it was directly put into the next reaction.
[0110] The crude product obtained in the previous step (66.5 g, 184 mmol) was dissolved in TFA (150 mL) and stirred at room temperature. After 3 hours, TFA was spinned off, water was added to the residue, the pH of NaOH solution was adjusted to above 10, and extracted with ethyl acetate. The extract was concentrated and then column chromatographed to obtain 35.3 g of white solid, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com