D, L-guanosine analogs, preparation methods thereof and applications thereof
A kind of technology of guanosine and purine nucleoside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
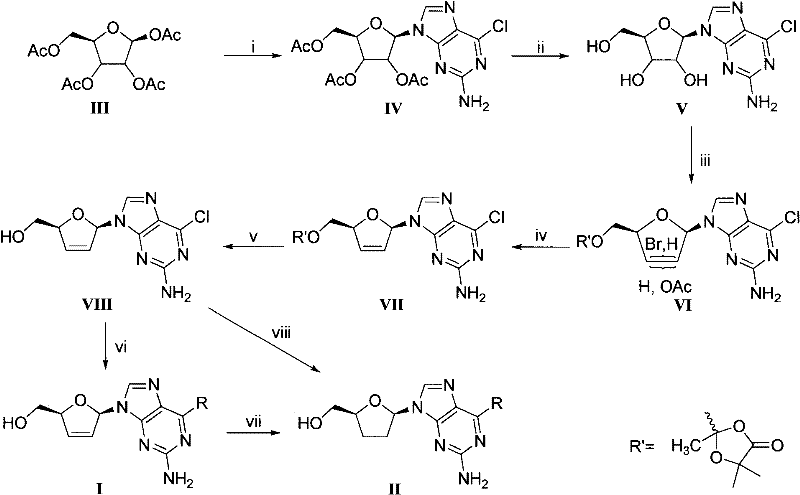
Image
Examples
Embodiment 1
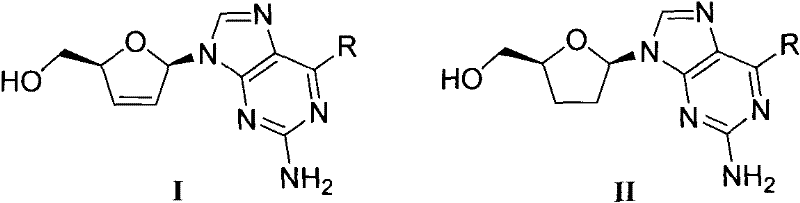
[0101] Example 1 The preparation of 2-amino-6-methylamino-9-2', 3'-dideoxydidehydro-β-D-purine nucleoside (D-Ia)
[0102] (1) Preparation of compound IV, 6-chloro-2 aminopurine base (1 g) was placed in a 50 mL three-neck flask, replaced with argon, and then dry DCE (10 mL) was added thereto; the system was in a suspended state at this time. Then BSA (2 mL) was added thereto, placed in an oil bath preheated to 80° C., and refluxed for 1 hour under the protection of argon, the system gradually became clear. The oil bath was removed and allowed to cool to room temperature, then a solution of D-tetraacetylribose (1 g) in DCE (4 mL) and TMSOTf (1 mL) was added thereto, heated to 80° C., and refluxed for 5 h under protection of argon. Remove the oil bath and pour it into ice saturated NaHCO 3 In the solution (20mL), bubbles and a large amount of sediment appeared. After the bubbling stopped, the precipitate was removed by filtration, extracted with DCM (20 mL), and the organic pha...
Embodiment 2
[0108] Example 2 Preparation of 2-amino-6-dimethylamino-9-2', 3'-dideoxydidehydro-β-D-purine nucleoside (D-Ib)
[0109] Suspend compound D-VIII (130mg, 0.486mmol) in ethanol (5mL), add aqueous dimethylamine solution (10mL), and reflux at 95°C for 1 hour. After the reaction of the raw materials is complete, the solvent is evaporated to dryness, and the sample is mixed with silica gel on an atmospheric pressure column. Separation (dichloromethane:methanol=120:1) gave white foamy solid product D-Ib (112mg), yield 88%. UV(MeOH) λ max 222nm (ε19214), 281.5 (ε12112); 1 H NMR (300MHz, CDCl 3 )δ7.51(s, 1H, 8-H), 6.67-6.70(m, 2H, 1'-H and OH), 6.376-6.41(m, 1H, 2'-H), 5.929-5.949(m, 1H, 3'-H), 5.07-5.08 (m 1H, 4'-H), 4.62 (brs, 2H, 2-NH 2 ), 4.06-4.01, 3.85-3.90 (m, 1H, 1H, 5'-H), 3.44 (brs, 6H, CH 3 ); 13 C NMR (125MHz, DMSO-d 6 )δ160.3, 155.4, 135.0, 134.0, 125.7, 113.4, 87.6, 87.2, 63.1, 62.8, 26.8; [α] D 20 -19.1 (c 0.18, MeOH).
Embodiment 3
[0110] Example 3 Preparation of 2-amino-6-cyclopropylamino-9-2', 3'-dideoxydidehydro-β-D-purine nucleoside (D-Ic)
[0111] Take compound D-VIII (100mg, 0.374mmol) and suspend it in ethanol (5mL), add cyclopropylamine (1mL), stir at room temperature for 36 hours, evaporate the solvent, and separate the sample under reduced pressure on silica gel (dichloromethane:methanol= 100:1) to obtain white foamy solid product D-Ic (89mg), yield 86%. UV(MeOH)λ max 224.5nm(ε16314), 284.5nm(ε12477); 1 H NMR (500MHz, DMSO-d 6 )δ7.72(s, 1H, 8-H), 7.32(brs, 1H, 6-NH), 6.75-6.76(m, 1H, 1'-H), 6.41-6.43(m, 1H, 2'- H), 6.07-6.09 (m, 1H, 3'-H), 5.85 (brs, 2H, 2-NH 2 ), 5.03-5.05(t, 1H, 5'-OH), 4.82-4.85(m, 1H, 4'-H), 3.54-3.60(m, 2H, 5'-H), 3.04(s, 1H, H-a), 0.560-0.68 (brs, 4H, H-b); 13 C NMR (125M Hz, DMSO-d 6 )δ160.2, 155.9 135.162, 134.0, 125.7, 113.3, 87.6, 87.2, 63.1, 6.4, 5.6; [α] D 20 -22.4 (c 0.21, MeOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com