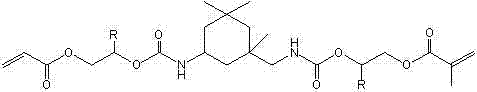
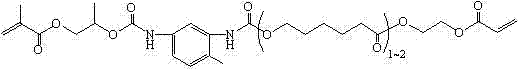
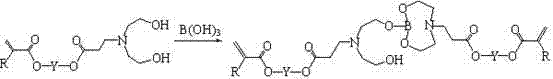
A kind of (meth)acrylated oxazole heterocyclic borate and its preparation method and application
A technology of methacrylate and heterocyclic boronate, which is applied in the field of (meth)acrylated nitrogen heterocyclic boronate and its preparation, and can solve the problem of lack of long carbon chain balance performance, unknown description, color deep questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Add 113.0 g (0.5 mol) of 1,6-hexanediol diacrylate into a three-necked flask, install a condenser tube, an addition funnel, and a mechanical stirrer, and place the flask in a cold water bath environment. Add 38.0 g (0.36 mol) diethanolamine dropwise under rapid stirring. After the dropwise addition of diethanolamine, continue to stir in a cold water bath environment for 6 hours, during which time samples are taken for NMR testing. It is confirmed that the acrylate in the system is 5.5~6.3 The relative intensity of the NMR signal peaks in the ppm interval no longer decreases, and the Michael addition reaction between the diethanolamine and the acrylate double bond is complete. Add 50 ml of toluene, 11.0 g (0.178 mol) of boric acid and 0.1 g of phenothiazine, replace the oil bath heating device, install a water separator, stir and heat to 95 °C, wait for the reflux to separate the water, and after the water separation begins, lower the temperature appropriately. Continue ...
Embodiment 2
[0111] Take 151.0 g (0.5 mol) of tripropylene glycol diacrylate and add it to a three-necked flask, install a condenser tube, an addition funnel, and a mechanical stirrer, and place the flask in a cold water bath environment. Add 31.5 g (0.3 mol) diethanolamine dropwise under rapid stirring. After the dropwise addition of diethanolamine, continue to stir in a cold water bath for 6 hours, during which time samples are taken for NMR testing. It is determined that the acrylate in the system is 5.5~6.3 The relative intensity of the NMR signal peaks in the ppm interval no longer decreases, and the Michael addition reaction between the diethanolamine and the acrylate double bond is complete. Add 70 ml of toluene, 9.2 g (0.15 mol) of boric acid and 0.1 g of phenothiazine, replace the oil bath heating device, install a water separator, stir and heat to 95 °C, wait for the reflux to separate the water, and after the water separation begins, lower the temperature appropriately. Continue...
Embodiment 3
[0113] Add 234.0 g (0.5 mol) of ethoxylated bisphenol A diacrylate SR 349 (Sartomer, molecular weight 468) into a three-neck flask, install a condenser tube, an addition funnel, and a mechanical stirrer, and place the flask in a cold water bath environment middle. Add 21.0 g (0.2 mol) diethanolamine dropwise under rapid stirring. After the dropwise addition of diethanolamine, continue to stir in a cold water bath environment for 6 hours, during which time samples are taken for NMR testing. It is confirmed that the acrylate in the system is 5.5~6.3 The relative intensity of the NMR signal peaks in the ppm interval no longer decreases, and the Michael addition reaction between the diethanolamine and the acrylate double bond is complete. Add 100 ml of toluene, 6.2 g (0.1 mol) of boric acid and 0.15 g of phenothiazine, replace the oil bath heating device, install a water separator, stir and heat to 95 °C, wait for the reflux to separate the water, and after the water separation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com