Preparation method of ammonia-containing composite ionic hydrogen storage material
A technology for hydrogen storage materials and composite ions, which is applied in the field of preparation of composite ion hydrogen storage materials, can solve the problems affecting the purity of hydrogen desorption, the effective degassing amount, the kinetics of dehydrogenation at low dehydrogenation temperature, and achieves moderate cost and easy preparation. , to achieve the effect of conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
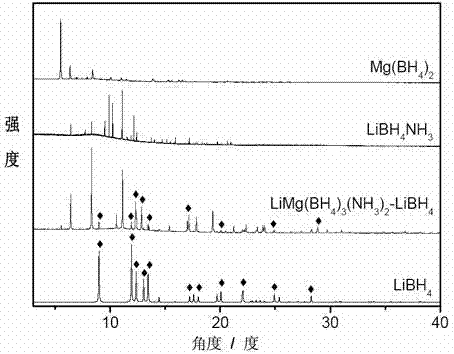
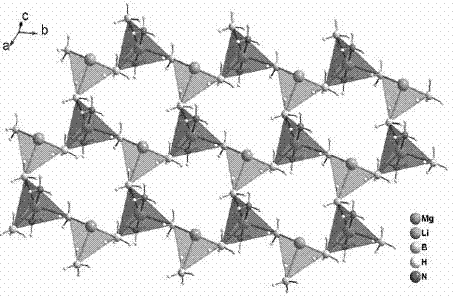
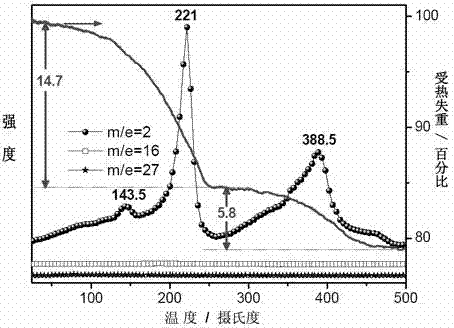
[0038] Example 1: At room temperature 25°C, 1g LiBH 4 Put it into a Schlenk test tube in argon, vacuumize the test tube, slowly feed ammonia gas, stop feeding ammonia gas when it reaches 0.8atm, and pump the test tube to a vacuum state after 20 minutes of reaction, and continue to vacuum for 3 hours Stop the reaction, take the product out under argon to obtain LiBH 4 ·NH 3 of white crystals. Take 0.78g LiBH in the glove box 4 ·NH 3 with 0.54g Mg(BH 4 ) 2 Mix, put into a ball mill jar, seal and take out the ball mill. The ball milling conditions are: the number of revolutions is 300-350 rpm, with stainless steel ball milling steel balls, the diameter is 0.5-2cm, the ball milling time is 6 hours, the operation mode is alternate pause and restart, the alternate time is 6 minutes, and the pause time is 6 minutes. After ball milling, Mg(BH 4 ) 2 / 2LiBH 4 ·NH 3 Compound, this compound is off-white powder. The results of high-resolution XRD test on the ball-milled sample ...
Embodiment 2
[0039] Example 2: At room temperature 25°C, 1g LiBH 4 Put it into a Schlenk test tube in argon, vacuumize the test tube, slowly feed ammonia gas, stop feeding ammonia gas when it reaches 0.8atm, and pump the test tube to a vacuum state after 20 minutes of reaction, and continue to vacuum for 3 hours Stop the reaction, take the product out under argon to obtain LiBH 4 ·NH 3 of white crystals. Take 0.39g LiBH in the glove box 4 ·NH 3 with 0.54g Mg(BH 4 ) 2 Mix, put into a ball mill jar, seal and take out the ball mill. The ball milling conditions are: the number of revolutions is 300-350 rpm, with stainless steel ball milling steel balls, the diameter is 0.5-2cm, the ball milling time is 6 hours, the operation mode is alternate pause and restart, the alternate time is 6 minutes, and the pause time is 6 minutes. After ball milling, a compound similar to Example 1 is obtained, and Mg(BH 4 ) 2 / LiBH 4 ·NH 3 express. This compound is also an off-white powder, and its hig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com