Isotope labeling reagent as well as preparation method and application thereof
A technology of isotope labeling and labeling reagents, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of erroneous results, low labeling efficiency, product mass spectrometry signal decline, etc., to reduce complexity, improve signal intensity, signal intensity and Stability-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Isotope labeling reagent of the present invention ([d 0 ] / [d 6 ]-DMPITC)
[0037] Dissolve 200mg of sodium metal in 20mL of anhydrous methanol / deuterated methanol to prepare a 20mL sodium methoxide / deuterated sodium methoxide solution;
[0038] Dissolve 500mg of 4,6-dichloro-2-aminopyrimidine in 10mL of anhydrous methanol / deuterated methanol, then add dropwise to the existing 20mL sodium methoxide / deuterated sodium methoxide solution, and place at 25°C stir overnight;
[0039] Concentrate by filtration, then dissolve in 10mL deionized water, extract 3 times with dichloromethane, 5mL each time, combine the organic phases, dry with sodium sulfate and spin dry to obtain [d 0 ] / [d 6 ]-4,6-dimethoxy-2-aminopyrimidine crude product;
[0040] 745 mg [d 0 ] / [d 6 The crude product of ]-4,6-dimethoxy-2-aminopyrimidine was dissolved in 20 mL of anhydrous dichloromethane, and thiophosgene (thiophosgene, 0.36 ml, 4.8 mmol) and sodium bicarbonate (1 g, 12 mmol) w...
Embodiment 2
[0044] Embodiment 2: Isotope labeling reagent of the present invention ([d 0 ] / [d 6 ]-DMPITC)
[0045] The principle of application is as follows:
[0046]
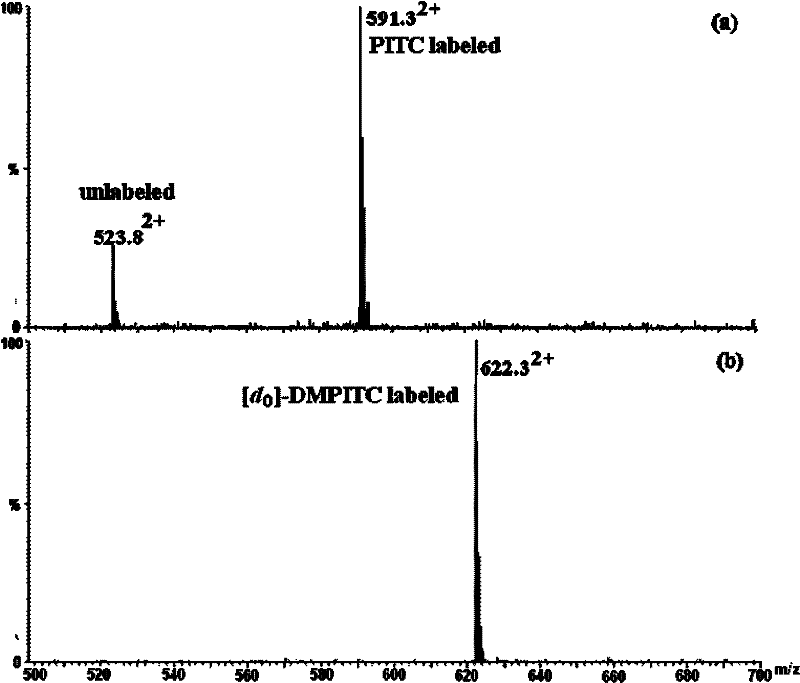
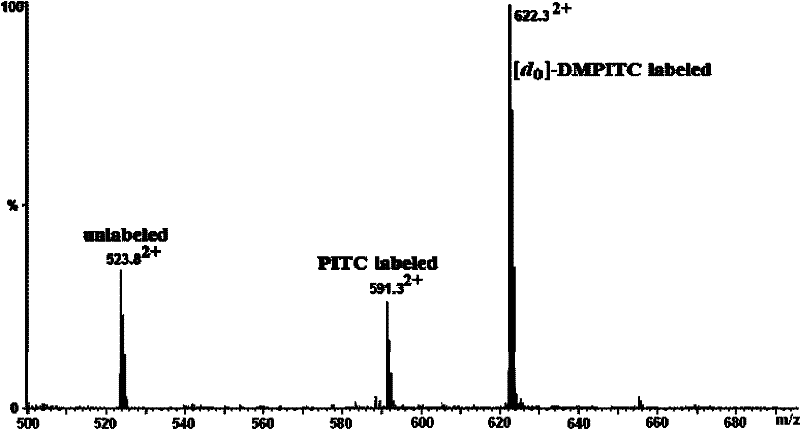
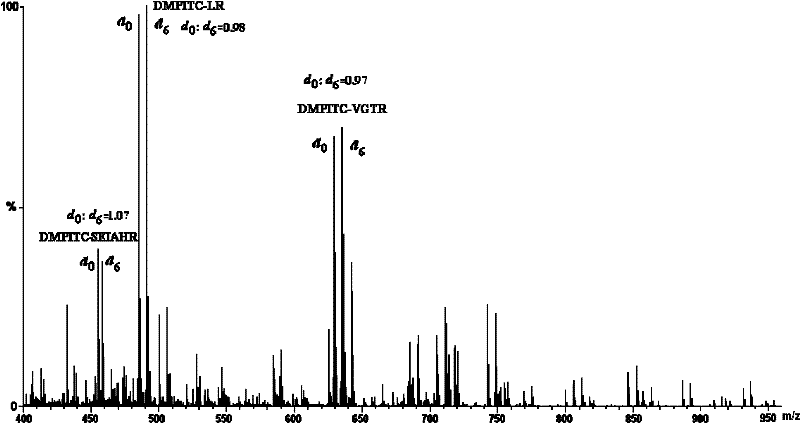
[0047] 1. Comparative labeling reaction of standard peptide (DRVYIHPF)
[0048] 1. Configure a mixed solution of ethanol / pyridine / water with a volume ratio of 2:2:1, and use this solvent as a reaction solvent;
[0049] 2. Add 400-600 μL of the above-mentioned reaction solvent to the two solutions of light-labeled reagents DMPITC and PITC (both 50 nmol / μL) dissolved in 100-200 μL of ethanol, and mix according to the molar ratio of labeling reagent and peptide segment of 500:1. Proportionally add the peptide solution, mix and react at 55-60°C for 90-120 minutes;
[0050] 3. After the reaction solution is cooled, blow it dry with nitrogen, then extract with dichloromethane to remove excess labeling reagent, then dry it with nitrogen, and dissolve the labeled peptide with 50-100 μL of acetonitrile-water solution with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com