Use of oxidoreductase or recombinase thereof and recombinant oxidoreductase
A reductase and reaction technology, applied in the field of recombinant oxidoreductase, can solve the problems of expensive coenzyme and high catalytic activity carbonyl reductase, etc., and achieve the effect of high optical purity, excellent catalytic activity and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
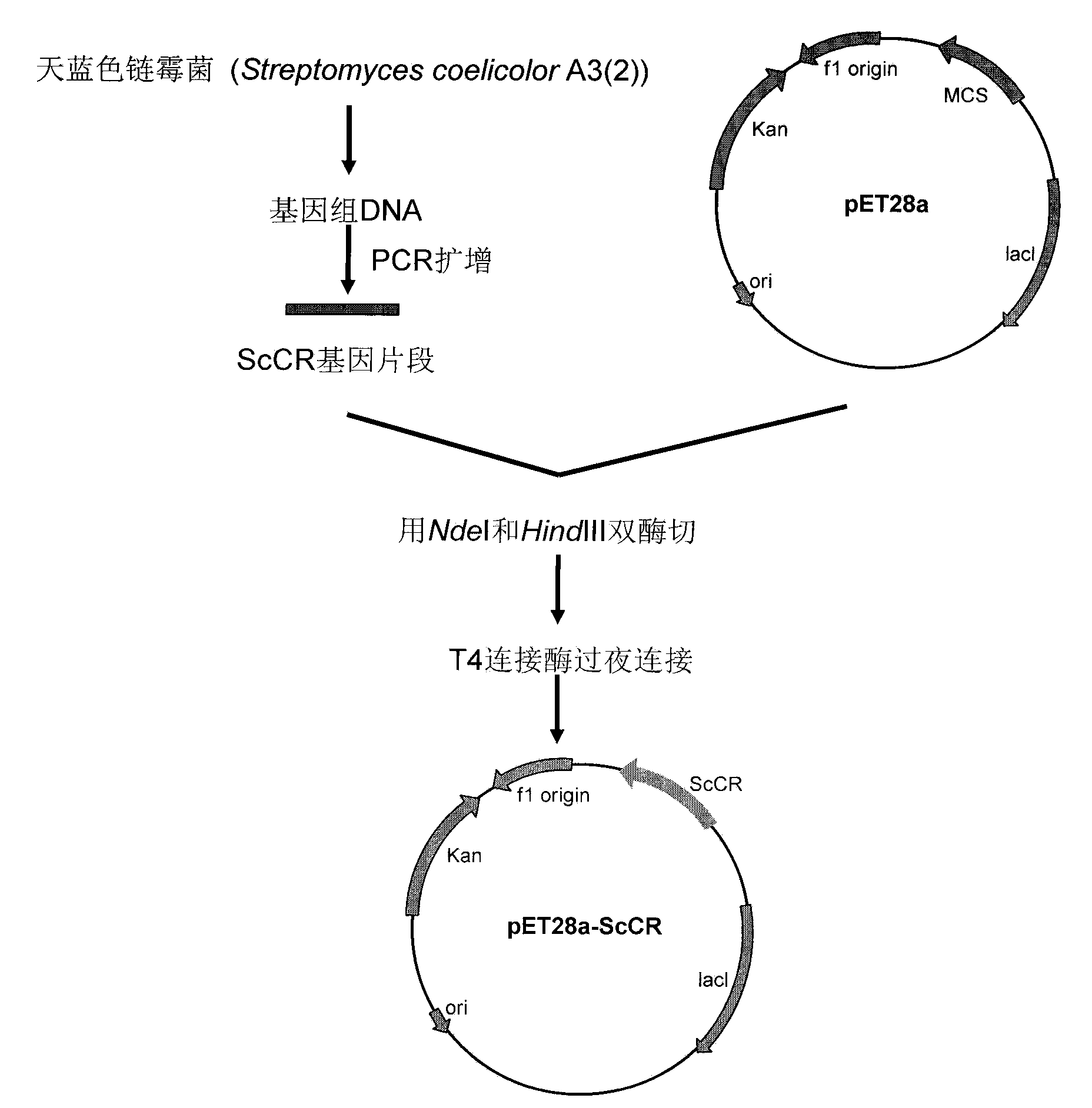
[0039] Example 1 Construction of Escherichia coli (E.coli) BL21(DE3) / pET-28a-ScCR
[0040] 1. Amplification of ScCR gene
[0041] According to the base sequence shown in SEQ ID No.1, the primer sequence is designed as follows:
[0042] Upstream primer (ScCR-NdeI): GGAGATT CATATG AGCACCACCGGAACCACC;
[0043] Downstream primer (ScCR-HindIII): GTG AAGCTT TCAGACGGCGGTGTAGGCGC.
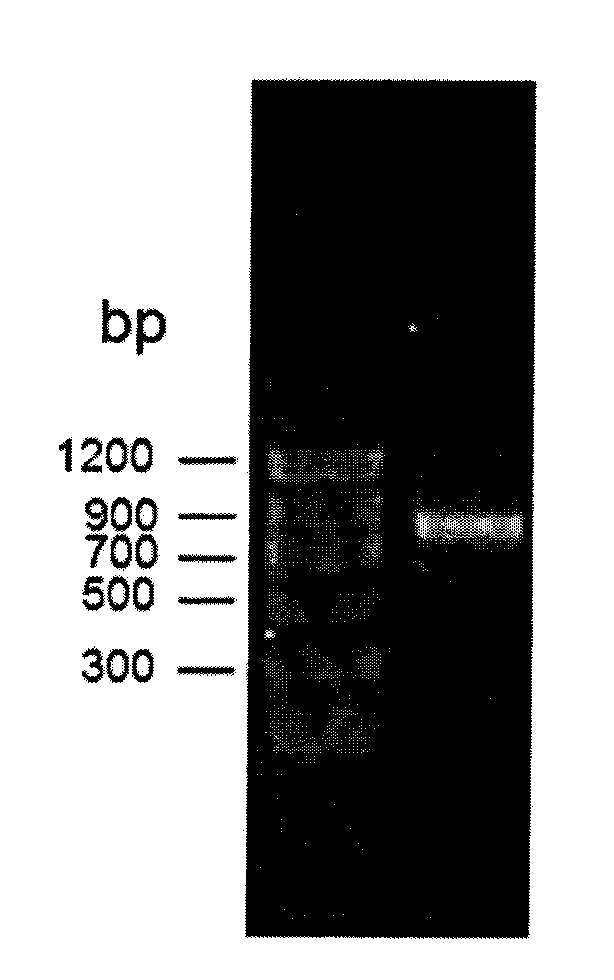
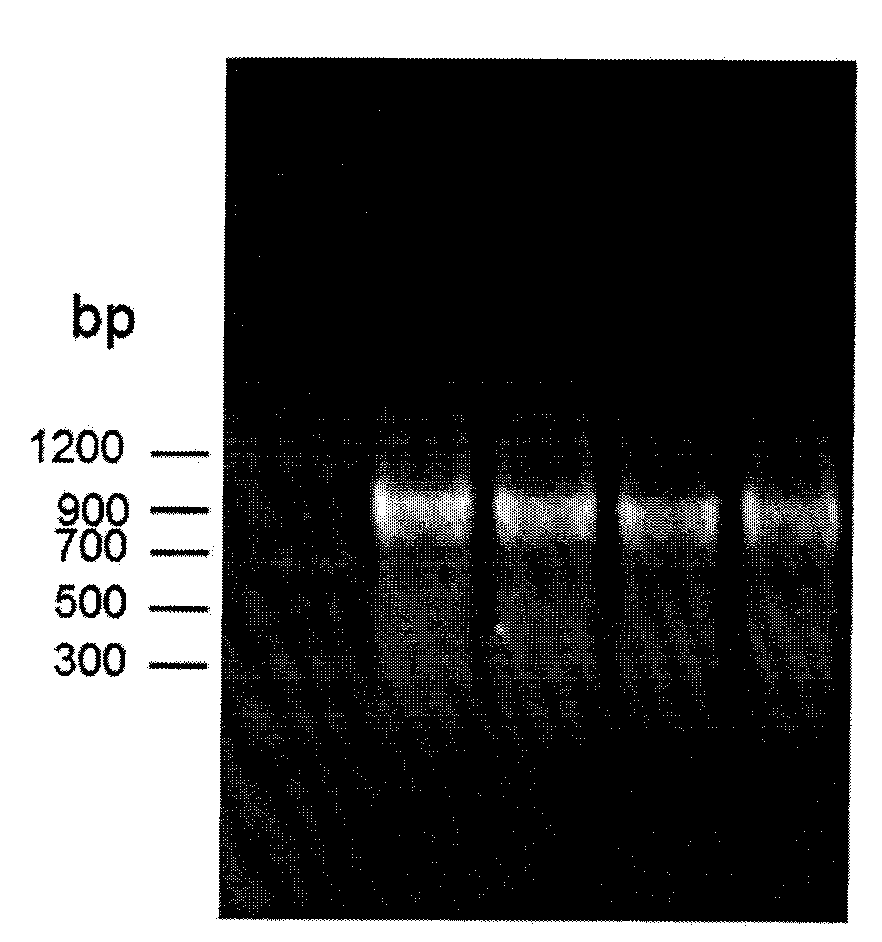
[0044] Using the genomic DNA of Streptomyces coelicolor A3(2) NRRL B-16638 as a template, PCR amplification was performed to obtain the ScCR gene. PCR system: 2×Taq PCRMasterMix 15μl, upstream primer and downstream primer 1μl (0.3μmol / L), DNA template 2μl (0.1μg) and ddH 2 O 11 μl. PCR amplification steps are: (1) 95°C, pre-denaturation for 3 minutes; (2) 94°C, denaturation for 1 minute; (3) 55°C annealing for 30s; (4) 72°C extension for 90s; steps (2) to (4) cycle 30 times; (5) 72°C, extended for 10min, cooled to 4°C for storage. The PCR product was purified by agarose gel electrophoresis, and t...
Embodiment 2
[0049] Example 2 Preparation of recombinant oxidoreductase ScCR
[0050]The Escherichia coli (E.coli) BL21 (DE3) / pET28a-ScCR that embodiment 1 gained is inoculated in the LB culture medium (comprising yeast extract 5g / L) containing kanamycin (final concentration 50mg / L, Peptone 10g / L and NaCl 10g / L), cultured with shaking at 37°C for 8h. Then by 5% (v / v) inoculum size, the seed liquid is inoculated in the 5L fermentor that 3L LB' medium (comprising yeast extract 10g / L, peptone 20g / L and NaCl 10g / L) is housed, 37 ℃ Grow to OD 600 At about 8 o'clock, IPTG with a final concentration of 0.5 mM was added and induced at 25°C for 12 hours.
[0051] The obtained fermentation culture liquid was centrifuged at 9000 rpm to remove the supernatant, and the wet cells were collected. Part of the wet cells were washed twice with physiological saline and freeze-dried to obtain freeze-dried cells for future use. Another part of wet cells was suspended in pH 6.5, 100mM phosphate-sodium phosp...
Embodiment 3
[0052] Example 3 Activity determination of recombinant oxidoreductase ScCR
[0053] Determination of the reduction reaction activity of the oxidoreductase ScCR: the reaction system is 1ml, including pH 6.5, 100mM sodium phosphate buffer, 0.1mM NADH, 2mM COBE, react at 30°C, and use a spectrophotometer to measure the decrease in absorbance at 340nm. Enzyme activity is defined as the amount of enzyme required to oxidize 1 μmol NADH per minute as one enzyme activity unit U. Protein content was determined by the Brandford method.
[0054] The above method was used to measure the activity of the recombinant oxidoreductase obtained in Example 2, and the result was that the reduction reaction activity of the recombinant oxidoreductase ScCR was 10.4 U / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com