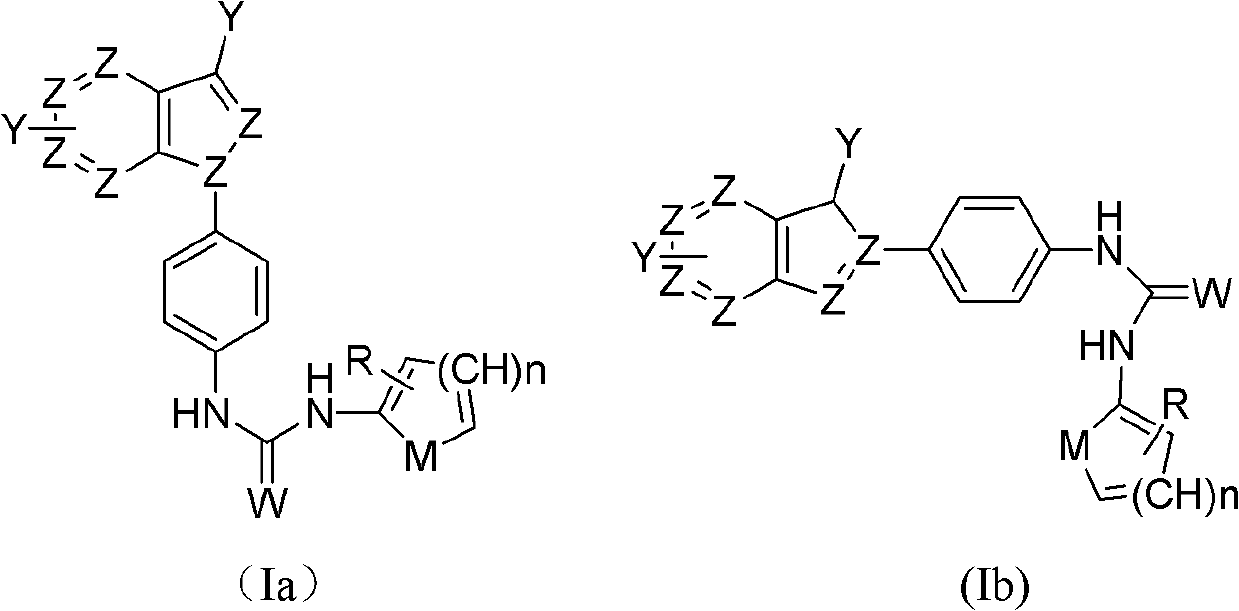
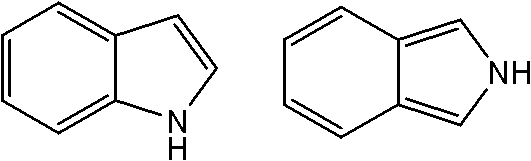
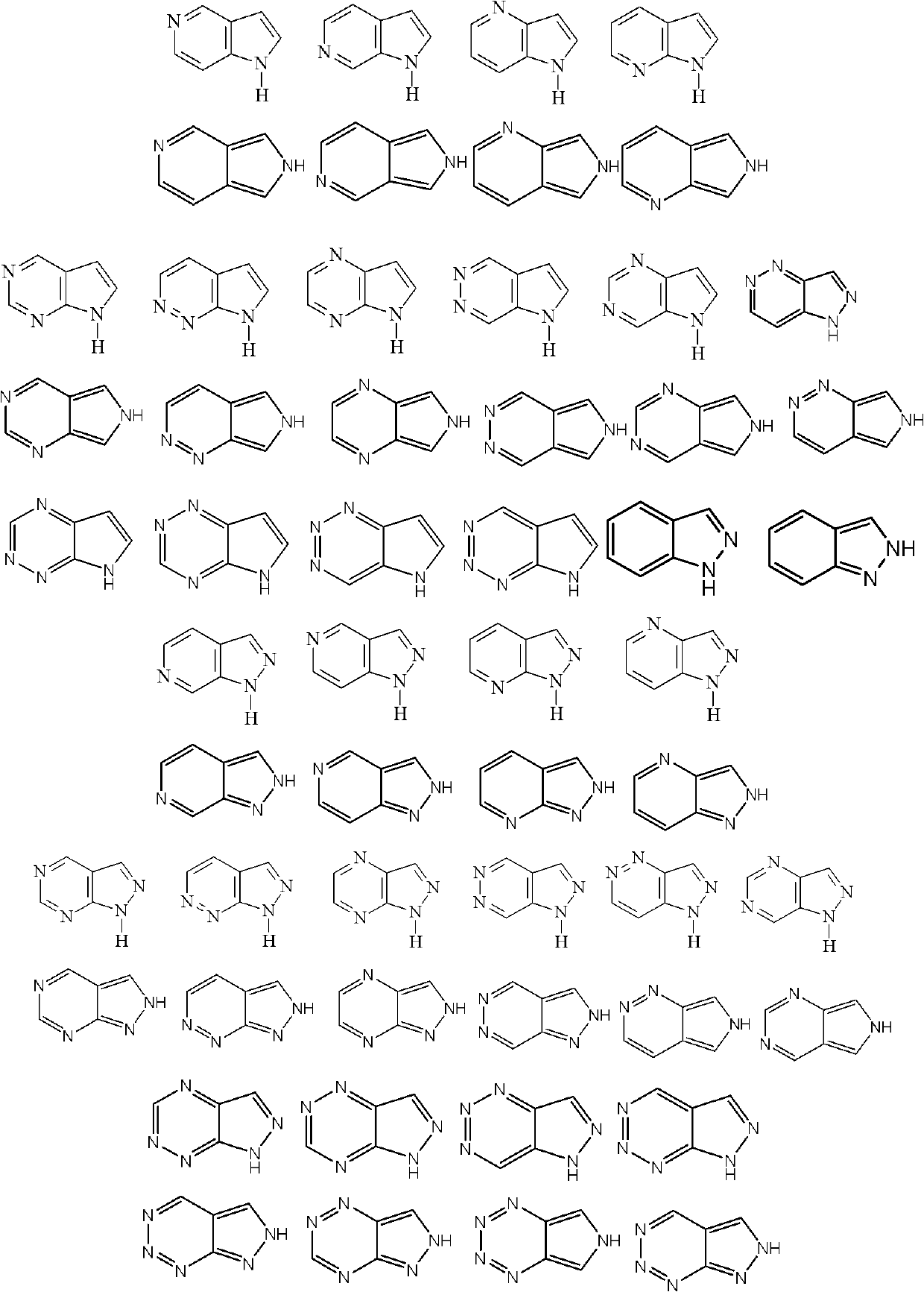
Indazole/azaindazole-based diarylcarbamide/thiocarbamide-structure antineoplastic drug
An anti-tumor drug, the technology of heteroindazole, applied in the field of anti-tumor drugs, can solve the problems that have not yet been discovered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: N-{4-[1-(5-azaindazole)]phenyl}-N'-[4-chloro-3-trifluoromethylphenyl]urea (1):
[0083]
[0084] 5-azaindazole (0.96g, 8.0mmol), p-fluoronitrobenzene (1.13g, 8.0mmol), cesium carbonate (5.2g, 16.0mmol) were dissolved in DMF (20ml), stirred overnight at room temperature, and The reaction solution was poured into water for filtration, and the filter cake was washed with water to obtain the target product, which was directly used in the next reaction without purification.
[0085] SnCl 2 7.5g (32.0mmol) was dissolved in 70mL concentrated hydrochloric acid, 1.9g (8.0mmol) of nitrophenyl-5-azaindazole obtained in the above step was added, and the reaction was stirred overnight at room temperature. Adjust the pH to 9 with saturated sodium bicarbonate solution, and extract with dichloromethane. The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. Purified by column chromatography (ethyl acetate:petroleum ether=2:1...
Embodiment 2
[0087] Example 2: N-{4-[1-(5-azaindazole)]phenyl}-N'-[4-chloro-3-trifluoromethylphenyl]thiourea (2):
[0088]
[0089] Dissolve 6.4g (33mmol) of 4-chloro-3-(trifluoromethyl)aniline and 11.2g (100mmol) of triethylenediamine in 40mL of toluene, then cool to 0°C, and slowly add CS 2 7.6g (100mmol), stirred at room temperature for 10h, filtered, and the filter cake was washed with toluene. Dissolve the filter cake in 40 mL CHCl 3 , and then dropwise added triphosgene (BTC) 3.3g (11.0mmol) dissolved in 15ml CHCl at 0°C 3 The solution was stirred at room temperature for 1h, and then refluxed for 1h. Cool to room temperature and filter. The filtrate was concentrated, and column chromatography (DCM:PE=1:1) gave 3.0 g of 4-chloro-3-trifluoromethylphenyl isothiocyanate as a yellow oily liquid, with a yield of 38.5%.
[0090] Referring to the method of Example 1, 1-(4-aminophenyl)-5-azaindazole was synthesized. Dissolve 100mg (0.49mmol) of the above 1-(4-aminophenyl)-5-azaindazol...
Embodiment 3
[0091] Example 3: N-{[4-[1-(6-azaindazole)]phenyl}-N'-[4-chloro-3-trifluoromethylphenyl]urea (3):
[0092] With reference to the method in Example 1, 9.6 g (40.0 mmol) of p-nitrophenyl-6-azaindazole was used to replace p-nitrophenyl-5-azaindazole, and the ratio of other raw materials and the operation method were the same , 5.4 g of 1-(4-aminophenyl)-6-azaindazole was obtained, with a yield of 70%. With reference to the method in Example 1, 1-(4-aminophenyl)-5-azaindazole was replaced with 1-(4-aminophenyl)-6-azaindazole (100mg, 0.49mmol), other Raw material proportioning and operating method are identical, obtain N-{[4-[1-(6-azaindazole)] phenyl}-N'-[4-chloro-3-trifluoromethylphenyl] urea ( 3) 170 mg, yield 81%. HPLC: 100%, LC-MS (C 20 h 13 CIF 3 N 5 O, MW=431): M+1=432; See Table-1 for relevant spectral data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com