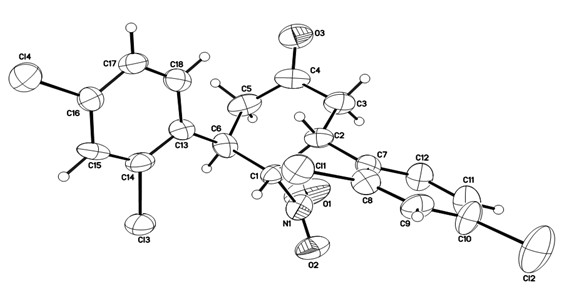
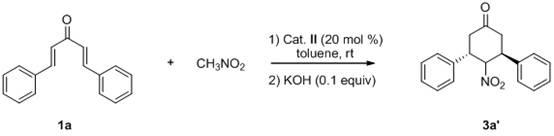
Novel method for synthesizing chiral 4-nitryl-3, 5-diaryl cyclohexanone
A cyclohexanone and chiral technology, applied in the field of asymmetric catalysis, can solve the problems of expensive raw materials and difficult to obtain prices, and achieve the effects of high yield, excellent diastereoselectivity and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] 1a (58.5 mg, 0.25 mmol), nitromethane (263.2 uL, 5.0 mmol) and thiourea catalyst (29.7 mg, 0.05 mmol) were successively charged into the reaction flask, and 1 mL of toluene was added, and the reaction was carried out at room temperature for 24-72 hours. After column chromatography (eluent: ethyl acetate:petroleum ether=1:5-1:10) to separate the starting material and catalyst, the residue was dissolved in potassium hydroxide (1.1 mg, 0.020 mmol) in ethanol (2 mL) In, react at 0 DEG C for 2 hours, obtain crude product;
[0041] The reaction system was quenched with saturated ammonium chloride, extracted with ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, and the solvent was removed. The crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10 ) to obtain the target product 3a (63.5 mg) with a yield of 86%.
[0042] The product is analyzed and the results are as follows: 1 H NMR (400 MH...
Embodiment 2
[0044]
[0045] 1a (58.5 mg, 0.25 mmol), nitromethane (263.2 uL, 5.0 mmol) and thiourea catalyst (29.7 mg, 0.05 mmol) were successively charged into the reaction flask, and 1 mL of toluene was added, and the reaction was carried out at room temperature for 24-72 hours. After column chromatography (eluent: ethyl acetate:petroleum ether=1:5-1:10) to separate the starting material and catalyst, the residue was dissolved in potassium hydroxide (1.1 mg, 0.020 mmol) in ethanol (2 mL) In, react at 0 DEG C for 2 hours, obtain crude product;
[0046] The reaction system was quenched with saturated ammonium chloride, extracted with ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, and the solvent was removed. The crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10 ) to obtain the target product 3a' (28.1 mg), with a yield of 38%.
[0047] The product is analyzed and the results are as follows: 1 H NMR (400 ...
Embodiment 3
[0049]
[0050] 1b (88.5 mg, 0.25 mmol), nitromethane (263.2 uL, 5.0 mmol) and thiourea catalyst (29.7 mg, 0.05 mmol) were successively charged into the reaction flask, and 1 mL of toluene was added, and the reaction was carried out at room temperature for 24-72 hours. After column chromatography (eluent: ethyl acetate:petroleum ether=1:5-1:10) to separate the starting material and catalyst, the residue was dissolved in potassium hydroxide (1.1 mg, 0.020 mmol) in ethanol (2 mL) In, react at 0 DEG C for 2 hours, obtain crude product;
[0051] The reaction system was quenched with saturated ammonium chloride, extracted with ethyl acetate, the organic layer was dried over anhydrous sodium sulfate, and the solvent was removed. The crude product was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10 ) to obtain the target product 3b (79.8 mg), with a yield of 77%.
[0052] The product is analyzed and the results are as follows: 1 H NMR (300 M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com