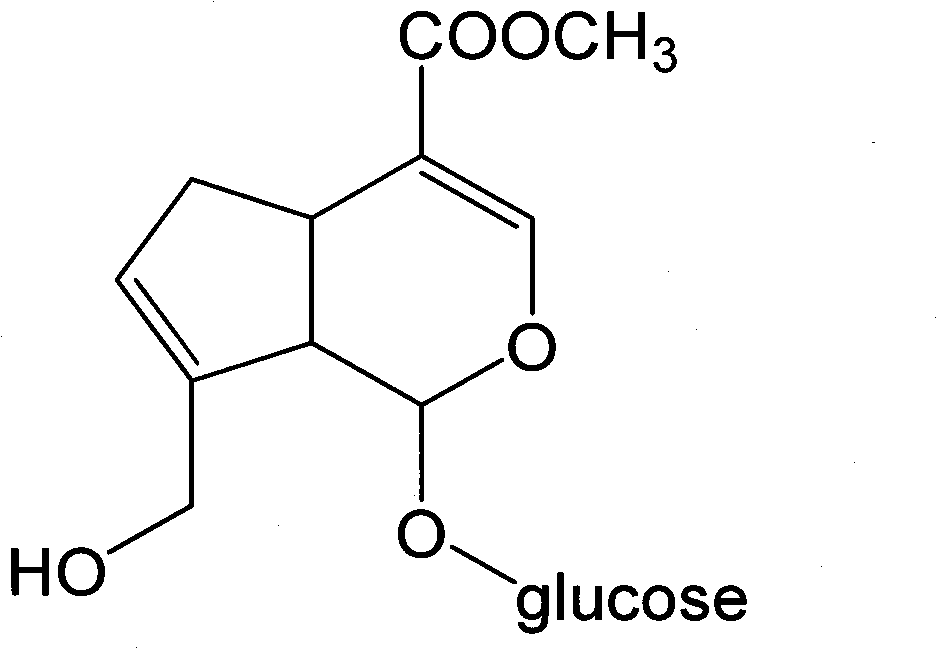
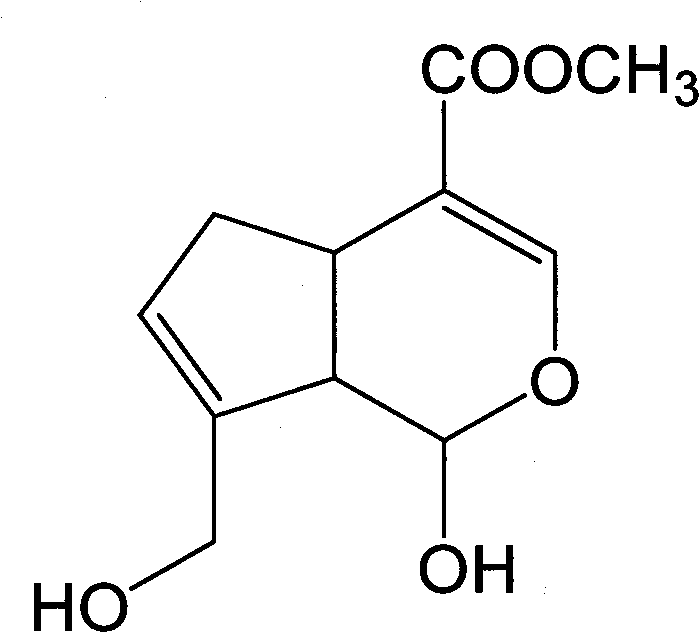
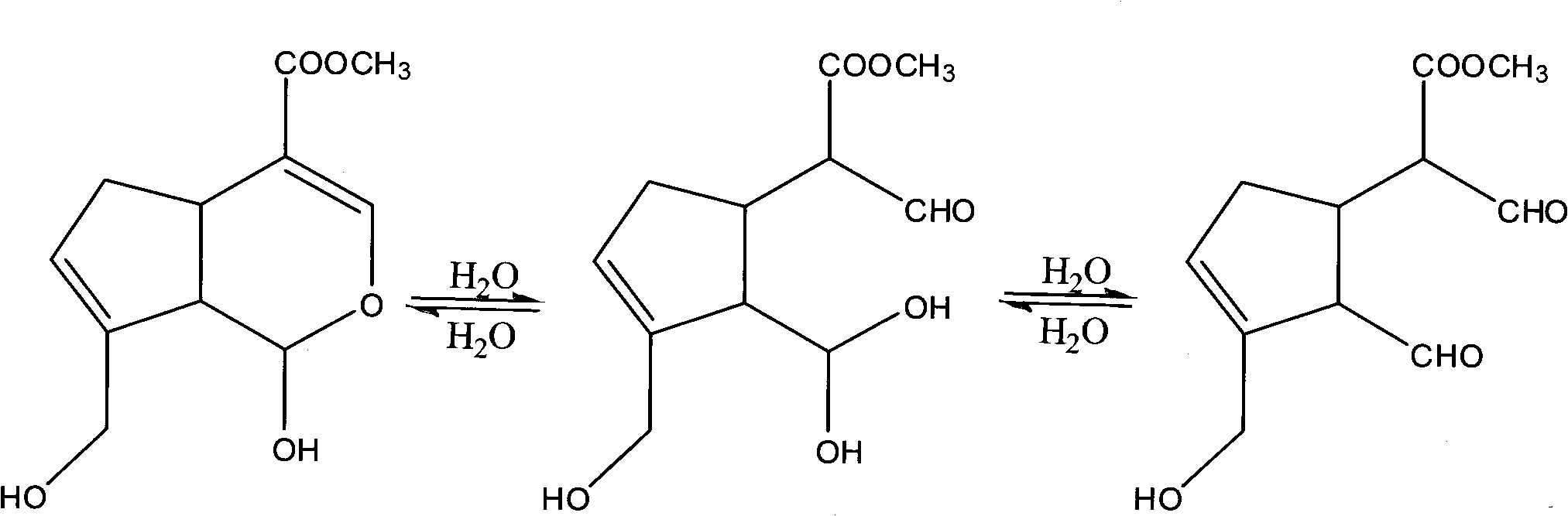
Method for preparing genipin
A technology of geniposide and glycoside hydrolase, which is applied in the direction of sustainable manufacturing/processing, immobilized on/in organic carriers, chemical industry, etc., and can solve the problem of industrial production of geniposide, β-glucose, etc. Problems such as high price of glucosidase and high dosage of β-glucosidase achieve the effect of high market price, difficulty in obtaining and reducing the generation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The reaction of free-glucosidase single-phase system hydrolyzing geniposide, the experimental operation steps are as follows:
[0039] (1) Take 0.5 g of geniposide with a purity of 90%, put it in a round bottom flask, add 50 U of free β-glucosidase, 50 ml of acetic acid-sodium acetate buffer solution (pH 4.5), heat and stir in a water bath at 55°C for 2 hours.
[0040] (2) The reaction solution was extracted three times with 50 ml of ethyl acetate, and the ethyl acetate extracts were combined.
[0041] (3) Add an appropriate amount of anhydrous sodium sulfate to the combined ethyl acetate extracts, dry for 30 minutes, and filter.
[0042] (4) Ethyl acetate was recovered by rotary evaporation under reduced pressure, and concentrated to 5% of the original volume.
[0043] (5) Put the concentrated liquid at 0°C for crystallization, and recover the crystals.
[0044] (6) The crystals were vacuum-dried at 40° C. for 1 h, weighed and stored.
[0045] Experimental results: ...
Embodiment 2
[0047] The reaction of free β-glucanase biphasic system to hydrolyze geniposide, the experimental steps are as follows:
[0048] (1) Take 0.5g of geniposide with a purity of 90%, put it in a round bottom flask, add 45U of free β-glucanase, 50ml of acetic acid-sodium acetate buffer solution (pH 4.2) and 50ml of ethyl acetate, at 50°C Heated and stirred in a water bath for 2h.
[0049] (2) The reaction solution was extracted three times with 50 ml of ethyl acetate, and the ethyl acetate extracts were combined.
[0050] (3) Add an appropriate amount of anhydrous sodium sulfate to the combined ethyl acetate extracts, dry for 30 minutes, and filter.
[0051] (4) Ethyl acetate was recovered by rotary evaporation under reduced pressure, and concentrated to 5% of its original volume.
[0052] (5) Put the concentrated liquid at 0°C for crystallization, and recover the crystals.
[0053] (6) The crystals were vacuum-dried at 40° C. for 1 h, weighed and stored.
[0054] Experimental re...
Embodiment 3
[0056] Immobilized β-glucanase single-phase system hydrolyzes geniposide, and the experimental steps are as follows:
[0057] (1) Weigh 0.6g of sodium alginate and add 20ml of deionized water, heat to dissolve.
[0058] (2) Add 110 U of free β-glucanase in proportion and mix well.
[0059] (3) Then add 0.64 ml of 25% glutaraldehyde aqueous solution, stir and cross-link at room temperature for 2 hours.
[0060] (4) Use a syringe (No. 5 needle) to draw the above solution, drop it into 100ml of calcium chloride solution with a concentration of 0.5% at a height of about 10cm, form a smooth gel ball with a diameter of about 3mm, and let it stand to harden 2h.
[0061] (5) The immobilized enzyme gel particles were filtered out, washed with deionized water, blotted to dry the surface moisture, and stored in a refrigerator at 4°C.
[0062] (6) Take 0.25g of geniposide with a purity of 90%, put it in a round bottom flask, add 5U of immobilized β-glucanase, 25ml of acetic acid-sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com