Antibody to beta-amyloid peptide and application thereof
A technology of amyloid peptide and antibody, applied in the direction of antibody, application, anti-animal/human immunoglobulin, etc., can solve the problems of low antibody affinity and inability to solve the source of human B cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
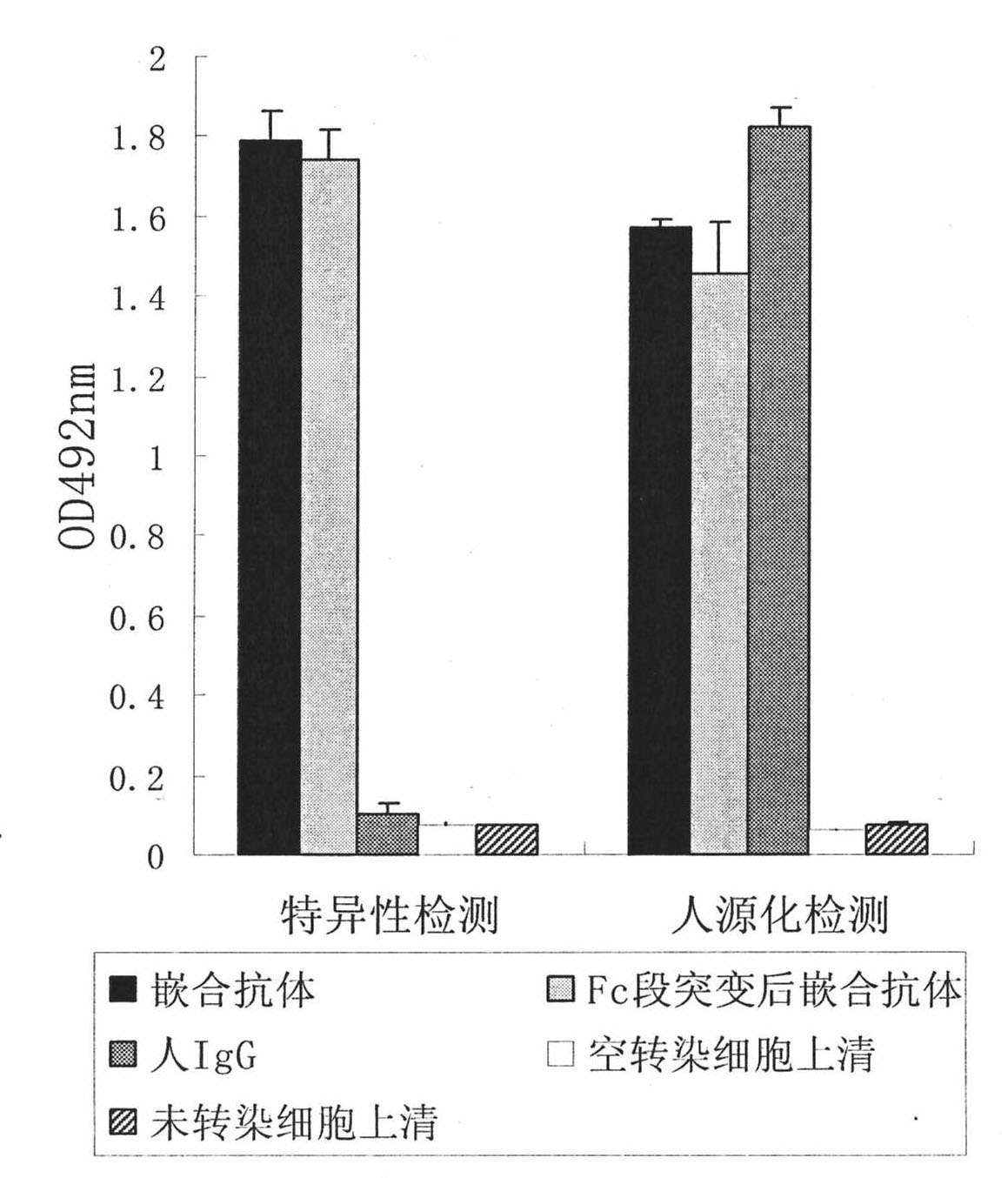
[0034] Example 1: Preparation and Identification of Anti-Aβ42 Monoclonal Antibody:
[0035] Aβ1-42 polypeptide 100μg / 0.1ml PBS (0.01M, PH7.2) was mixed with an equal volume of Freund's complete adjuvant (CFA) as an immunogen, mixed with each other through a syringe until emulsified, and subcutaneously in the abdomen (6 points) ) to immunize female BALB / c mice for 5-6 weeks, and inject 200 μl per mouse; after the basic immunization, repeat the injection 5 times every two weeks to boost the immunization and monitor changes in serum antibody levels; select the sensitized mice with the highest titer , Three days before cell fusion, Aβ1-42 polypeptide 50μg / 0.1ml saline was injected into the tail vein, and cell fusion was carried out 10 days later. Spleen single cells were routinely isolated, and spleen cells from immunized mice (1×10 8 ) and SP2 / 0 myeloma cells (1×10 7 ) and mix, centrifuge at 1000rpm for 5 minutes, suck up the supernatant and flick the bottom of the tube to loos...
Embodiment 2
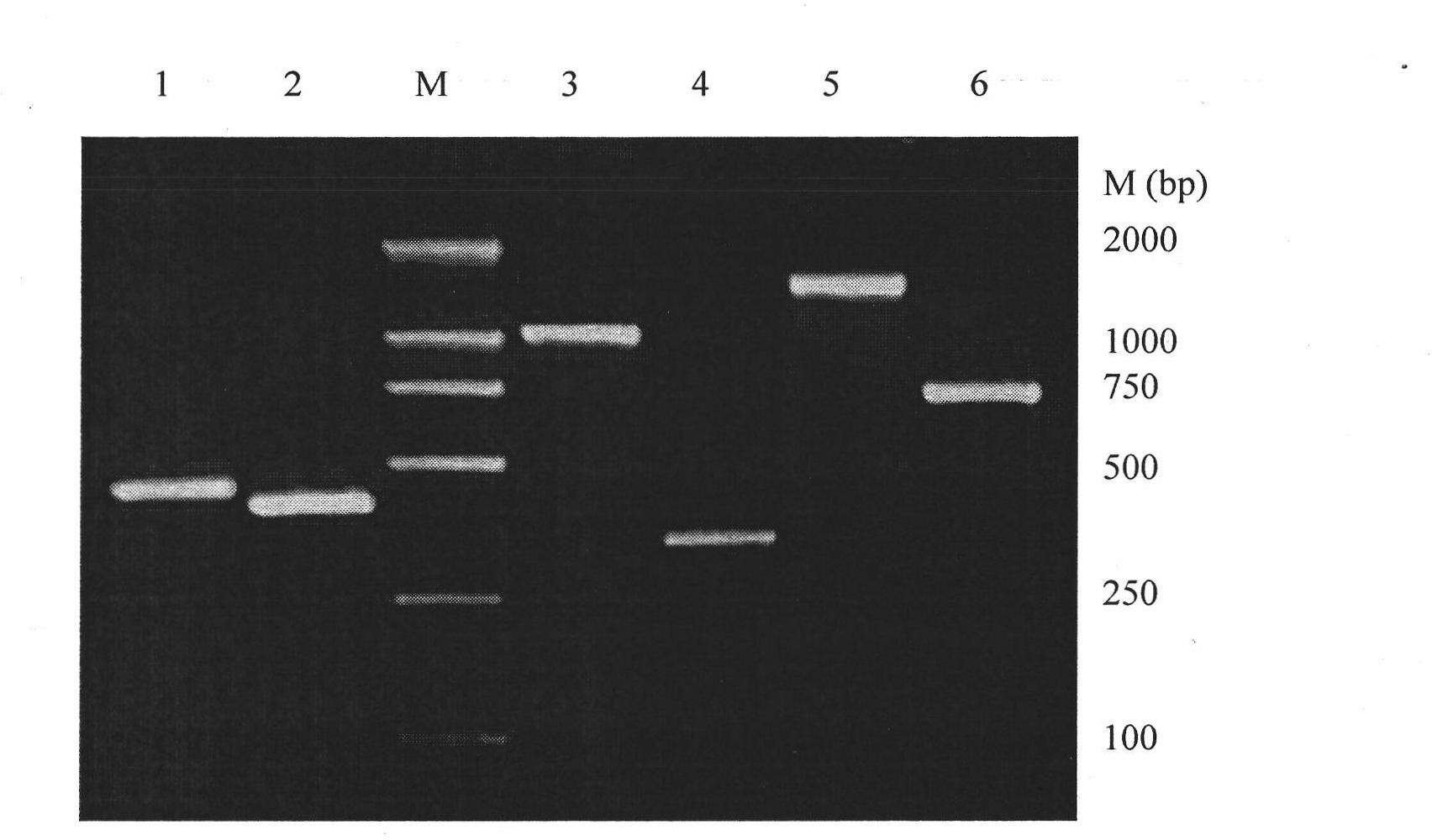
[0036] Example 2: Amplification and sequencing of the variable region of the anti-Aβ42 monoclonal antibody
[0037] Adopt BD SMART TM The 5'-RACE method in the RACE cDNA kit was used to obtain the variable region coding sequence of the anti-Aβ1-42 mouse monoclonal antibody mZ1, and the primers were designed as follows:
[0038] GSP-L1: 5'-CTATGAATTCTCAACACTCATTCCTGTTGAAGC-3'
[0039] GSP-L2: 5'-CTATGAATTCTCAGRRACAKTCWGCASGRGACA-3'
[0040] GSP-H: 5'-CTATGAATTCTCATTTACCAGGAGAGTGGGAGAG-3'
[0041] One-step extraction of 1×10 with TRIZOL 7 Total RNA of hybridoma mZ1. According to the 5'-RACE kit instructions (BD Company), the first strand was amplified with 5'-CDS and BD SMART II oligo as primers, and then poly(C) was added to the 3' end of the first strand cDNA After tailing, the mouse antibody gene sequence was amplified with the universal primer UPM and the gene-specific primer GSP. The reaction conditions are: 94°C for 30 seconds, 72°C for 3 minutes, 5 cycles; 94°C for...
Embodiment 3
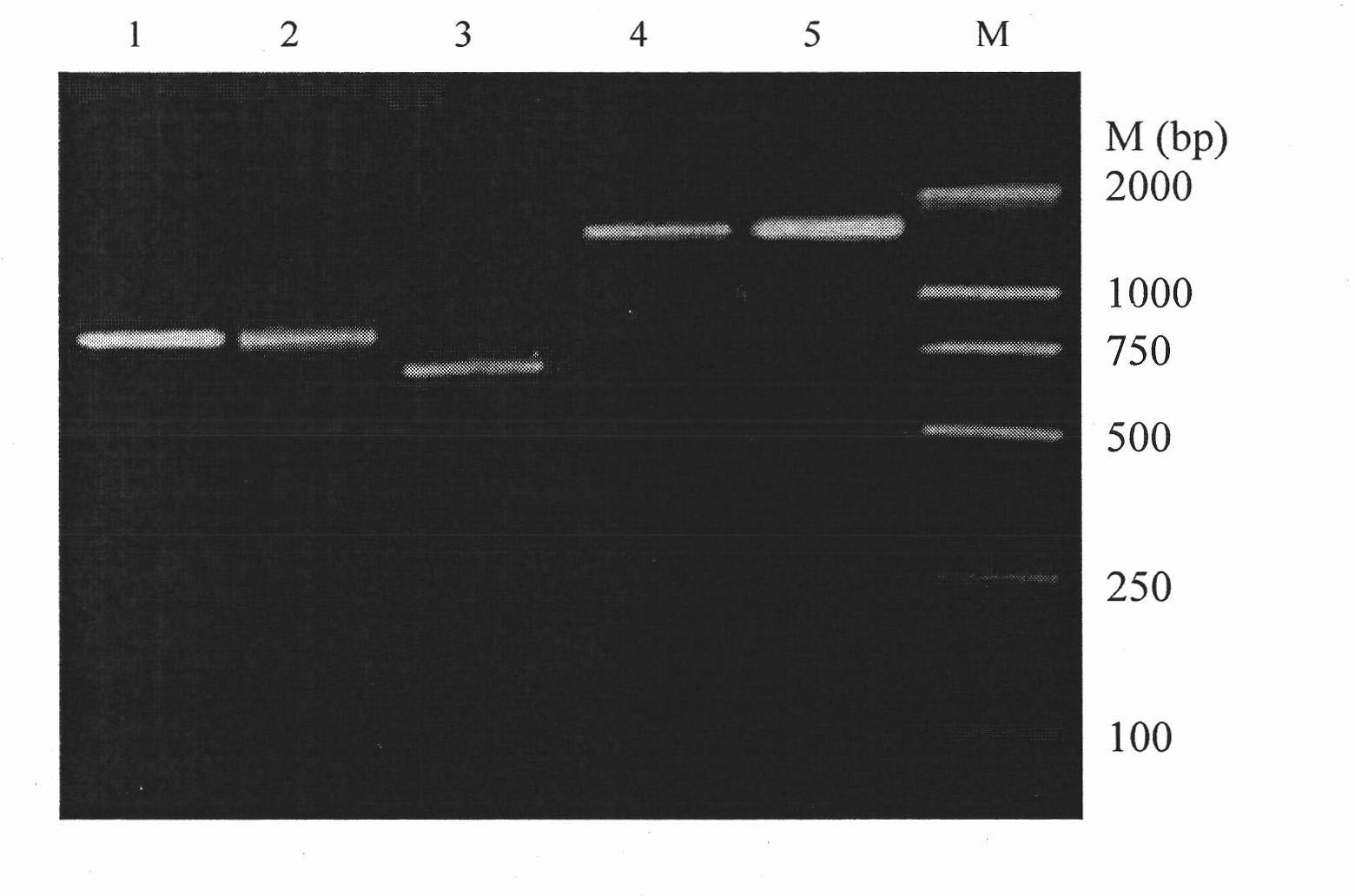
[0046] Example 3: Construction of Humanized Chimeric Monoclonal Antibody Expression Vector
[0047] The heavy and light chain variable regions of the mouse monoclonal antibody mZ1 were recombined with the human IgG antibody constant regions by recombinant PCR to form a full-length antibody. At the same time, the rare amino acids at the end of the heavy and light chains were mutated when designing primers, and the pre-designed primers were used. The restriction site of the enzyme was connected into the expression vector of PCDNA3.1(+) and PCDNA3.1(-), wherein the restriction site at the 5' end was Hind III, and the restriction site at the 3' end was XbaI. The designed primers were as follows:
[0048] Light chain variable region forward primer VLf (23bp): 5'-cccAAGCTTcaccATGTCCTCTGCTCAGTTCCTTGG-3'
[0049] Light chain variable region reverse primer VLb (31bp): 5'-TGCAGCCACAGTTTTGATCTCTACCTTGGTG-3'
[0050] Light chain constant region forward primer CLf (31bp): 5'-GTAGAGATCAAAc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com