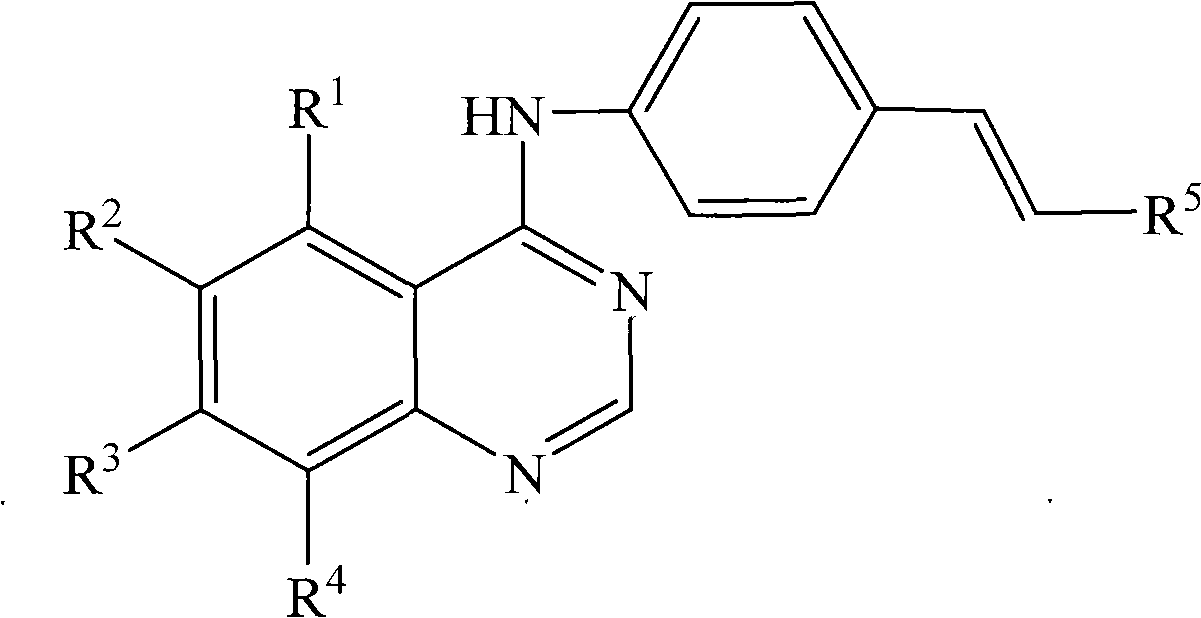
Novel anilinoquinazoline derivatives and preparation method thereof
A technology for aniline quinazoline derivatives, applied in the field of novel aniline quinazoline derivatives and their preparation, capable of solving problems such as strong toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the preparation of compound A
[0061]
[0062] Add 6-fluoro-4-chloroquinazoline (0.11g, 0.58mmol), (E)-3,4,5-trimethoxy-4'-aminostilbene (0.17g, 0.58mmol) and absolute ethanol (20mL). Heated to reflux for 1h, cooled, and filtered to obtain a bright yellow solid. The obtained solid was washed with a mixed solvent of ethanol and triethylamine until the impurities disappeared, then washed repeatedly with water, and suction-filtered to obtain 0.19 g of a bright yellow solid, namely the target compound, with a yield of 76%. m.p.: 255.0-255.2°C; 1 H NMR (300MHz, DMSO-d 6 )δ: 3.71 (s, 3H, OCH 3 ), 3.87(s, 6H, 2OCH 3 ), 6.94(s, 2H), 7.17-7.29(m, 2H), 7.62-7.95(m, 6H), 8.46(d, 1H), 8.65(s, 1H), 9.79(s, 1H, NH);
[0063] IR v max (KBr)cm -1 : 3618, 3333, 2991, 2935, 1634, 1609, 1577, 1507, 1422, 1343, 1243, 1127, 1006, 970 (trans, CH=CH);
[0064] Elemental analysis (%): C 25 h 22 FN 3 o 3 , found (calculated): C, 69.74 (69.59); H, 5.41 (5.14); N, 9...
Embodiment 2
[0065] Embodiment 2: the preparation of compound B
[0066]
[0067] Add 6-fluoro-4-chloroquinazoline (0.16g, 0.87mmol), (E)-3,4-dimethoxy-4'-aminostilbene (0.22g, 0.87mmol) successively in a 50mL three-necked flask ) and absolute ethanol (30mL). Heated to reflux for 2h, cooled, and filtered to obtain a yellow solid. The obtained solid was washed with a mixed solvent of ethanol and triethylamine until the impurities disappeared, then washed repeatedly with water, and suction filtered to obtain 0.23 g of a light yellow solid, namely the target compound, with a yield of 66%. m.p.: 265.5-267.1°C;
[0068] 1 H NMR (300MHz, DMSO-d 6 )δ: 3.78(s, 3H, OCH 3 ), 3.84(s, 3H, OCH 3 ), 6.94-7.25(m, 5H), 7.61(d, 2H), 7.78-7.9(m, 4H), 8.46(d, 1H), 8.63(s, 1H), 9.77(s, 1H, NH);
[0069] IR v max (KBr)cm -1 : 3351 (NH), 3051, 2933, 2835, 1630, 1600, 1522, 1419, 1280, 1237, 1140, 1024, 951 (trans, CH=CH);
[0070] Elemental analysis (%): C 24 h 20 FN 3 o 2 , found (calculated):...
Embodiment 3
[0071] Embodiment 3: the preparation of compound C
[0072]
[0073] Add 6-fluoro-4-chloroquinazoline (0.91g, 5.0mmol), (E)-3,5-dimethoxy-4'-aminostilbene (1.28g, 5.0mmol) into a 50mL three-necked flask ) and isopropanol (35 mL). Heat to reflux for 4h, add 1.0mL triethylamine, continue stirring for 0.5h, evaporate to dryness, add 10mL water, stir, and filter. The obtained solid was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:2, V / V) to obtain 1.15 g of light yellow solid with a yield of 57%. 1 H NMR (300MHz, DMSO-d 6 )δ: 3.80(s, 6H, 2OCH 3 ), 6.42(s, 1H), 6.79(s, 2H), 7.12-7.32(dd, 2H, J=16.2), 7.65-7.94(m, 5H), 8.10(s, 1H), 8.46(br s, 1H), 8.65(s, 1H), 9.79(s, 1H, NH);
[0074] 13 C NMR (75MHz, DMSO-d 6 )δ: 55.69, 100.24, 104.85, 107.73, 110.77, 122.55, 123.06, 127.20, 127.87, 129.05, 131.23, 132.91, 139.13, 139.77, 145.29, 147.35, 154.47, 157.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com