Conductive coating composition
A conductive polymer and composition technology, applied in the direction of conductive coatings, conductive materials, conductive materials, etc., can solve the problems of poor dispersion stability of inherent conductive polymers, inability to remove firmly adhered cations, water content reduced to below 1wt%, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0134] First, a UV light curable adhesive composition was prepared by mixing the components listed below.
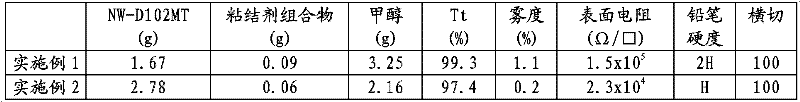
[0135] NW-D102MT, which is a dispersion of intrinsically conductive polymer containing doped polyaniline in methanol, solids content 0.9 wt%. Manufactured by Nissan Chemical Industries Ltd.
[0136] ACR-ST-2101 (9.5 g), which is colloidal silicon dioxide dispersed in tetrahydrofuryl acrylate, has a silicon dioxide content of 30 wt%. (same below). Produced by Nissan Chemical Industries Ltd.
[0137] KAYARAD PET30 (9.5 g), which is pentaerythritol triacrylate. Produced by Nippon Kayaku Co., Ltd.
[0138] IRGACURE 184 (1 g), which is a photoinitiator, manufactured by Ciba-Geigy Corporation.
[0139] Methanol (30g)
[0140] Next, the obtained binder was mixed with methanol for concentration adjustment at the mixing ratio shown in Table 1.
[0141] A coating composition was thus obtained, and its dispersion state was found to be good.
[0142] The coating composition ...
Embodiment 3 and 4
[0146] First, a UV light curable adhesive composition was prepared by mixing the components listed below.
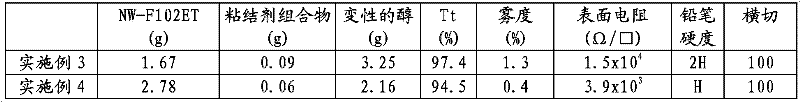
[0147] NW-F102ET, which is a dispersion of intrinsically conductive polymer containing doped polyaniline in methanol-denatured alcohol, solid content 0.9 wt%. Manufactured by Nissan Chemical Industries Ltd.
[0148] ACR-ST-2101 (9.5 g), which is colloidal silica dispersed in tetrahydrofuryl acrylate. Manufactured by Nissan Chemical Industries Ltd.
[0149] KAYARAD PET30 (9.5 g), which is pentaerythritol triacrylate. Produced by Nippon Kayaku Co., Ltd.
[0150] IRGACURE 184 (1 g), which is a photoinitiator, manufactured by Ciba-Geigy Corporation.
[0151] Methanol denatured alcohol (30g)
[0152] Next, the obtained binder was mixed with methanol-denatured alcohol for concentration adjustment at the mixing ratio shown in Table 2.
[0153] A coating composition was thus obtained, and its dispersion state was found to be good.
[0154] The coating composition thus obt...
Embodiment 5
[0158] First, a UV light curable adhesive composition was prepared by mixing the components listed below.
[0159] NW-F101MEK, which is a dispersion of intrinsically conductive polymer containing doped polyaniline in 2-butanone, solids content 0.9 wt%. Manufactured by Nissan Chemical Industries Ltd.
[0160] ACR-ST-2101 (9.5 g), which is colloidal silica dispersed in tetrahydrofuryl acrylate. Manufactured by Nissan Chemical Industries Ltd.
[0161] KAYARAD PET30 (9.5 g), which is pentaerythritol triacrylate. Produced by Nippon Kayaku Co., Ltd.
[0162] IRGACURE 184 (1 g), which is a photoinitiator, manufactured by Ciba-Geigy Corporation.
[0163] 2-Butanone (30g)
[0164] Next, the obtained binder was mixed with 2-butanone for concentration adjustment at the mixing ratio shown in Table 3.
[0165] A coating composition was thus obtained, and its dispersion state was found to be good.
[0166] The coating composition thus obtained was made into a conductive coating film ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com