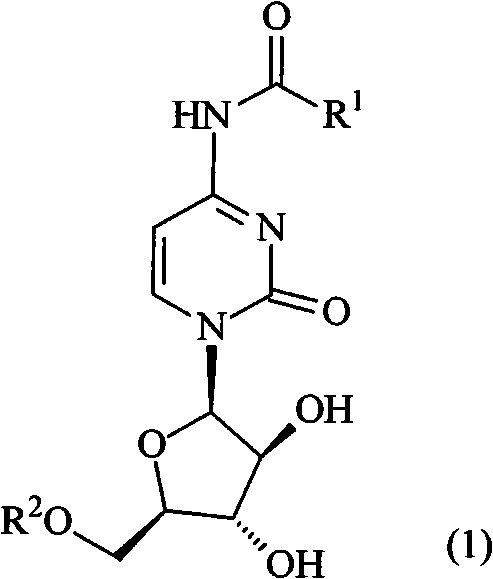
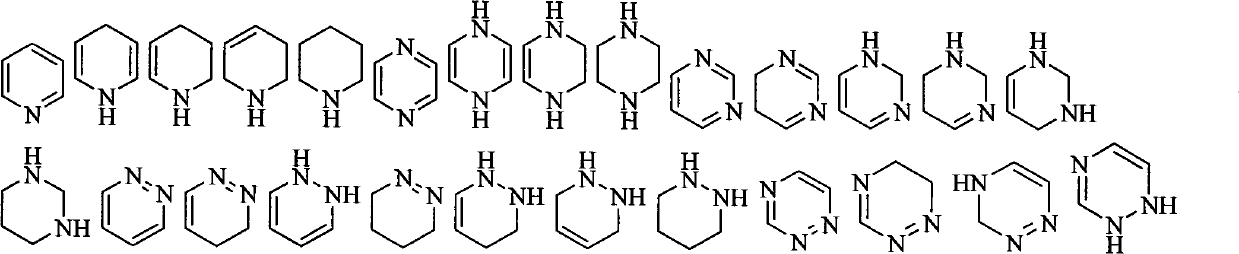
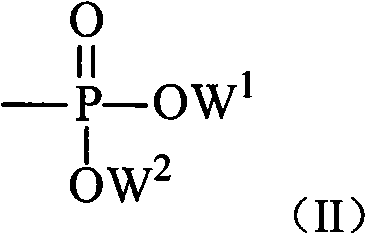Prodrug based on cytosine arabinoside structure, and synthesis method and application thereof
A kind of technology of cytarabine and synthetic method, applied in the application field of prodrug and drug based on cytarabine structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The present invention also provides the synthesis method of the prodrug based on the cytarabine structure, comprising the following steps:
[0064] 1) Acid anhydride or acid chloride and alcohol are directly mixed or dissolved in an organic solvent according to the ratio of acid anhydride or acid chloride: alcohol = 1: 1 to 1: 1.5 molar ratio, and reacted at room temperature to the melting temperature of the reactant for 2 to 8 hours to obtain the corresponding The substituted acid (ie R in the above formula 1 COOH);
[0065] 2) Cytarabine hydrochloride, the substituted acid obtained in step 1), benzotriazole-1-oxytripyrrolidinylphosphonium hexafluorophosphate (PyBOP) and 4-dimethylaminopyridine (DMAP) according to 1: (1~2): (0.9~1.5): (1~3) ratio of molar ratio dissolved in organic solvent, stirring at room temperature for 12~24 hours;
[0066] 3) Pour the reaction solution in step 2) into water, extract, and dry and purify the separated organic phase to obtain the t...
specific Embodiment approach
[0097] Hereinafter, the present invention will be described more specifically using examples, but the present invention is not limited to the following examples.
[0098] Cytarabine, purchased from Suzhou Surui Pharmaceutical Chemical Co., Ltd.;
[0099] Benzotriazole-1-oxytripyrrolidinylphosphine hexafluorophosphate was purchased from Jill Biochemical (Shanghai) Co., Ltd.;
[0100] 4-Dimethylaminopyridine was purchased from Jill Biochemical (Shanghai) Co., Ltd.
Embodiment 1
[0102] N 4 -(5-n-dodecyloxyformylthiophene-2-formyl)-cytarabine (compound NO.1)
[0103] 1.0 g of 2,5-thiophenedicarboxylic acid (5.8 mmol) was added to 15 mL of 8OCl 2 Add 2 drops of DMF and heat to reflux for 4 hours. spin off SOCl 2 , 1.21 g of thiophene-2,5-diacyl chloride was obtained almost quantitatively.
[0104] Dissolve 1.21g of thiophene-2,5-diacyl chloride (5.8mmol) in 30mL of dioxane, add 1.01g of lauryl alcohol and 2mL of Et 3 N 5mL dioxane solution, reacted for 4h, concentrated to dryness, the residue was dissolved in water, adjusted to pH 2-3, extracted three times with ethyl acetate, and the separated organic phase was dried with anhydrous sodium sulfate. After evaporation of the solvent under reduced pressure, 5-n-dodecyloxyformylthiophene-2-carboxylic acid was obtained almost quantitatively.
[0105] 406mg of cytarabine (1.67mmol), 683mg of 5-n-dodecyloxyformylthiophene-2-carboxylic acid (2.01mmol), 955mg of benzotriazole-1-oxytripyrrolidinylphosphonium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com