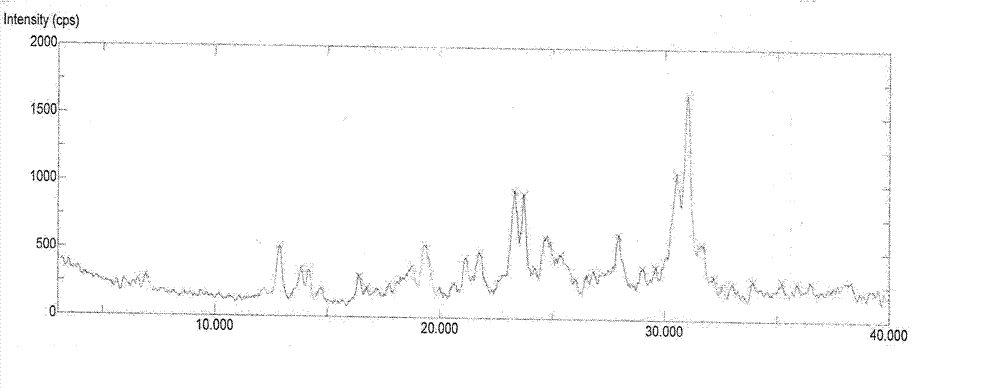
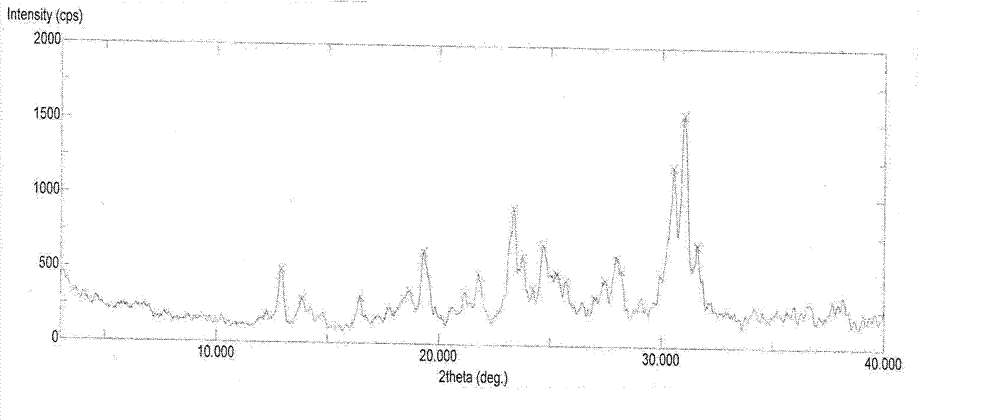
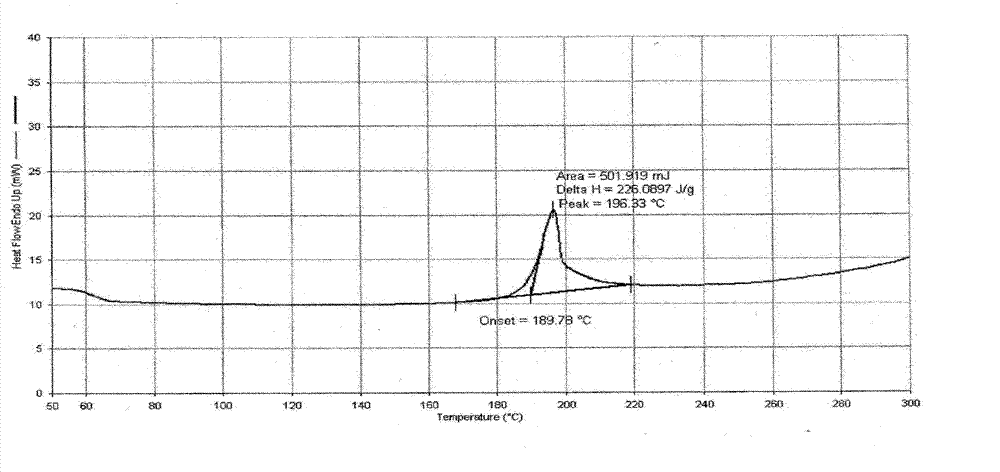
Decitabine lyophilized preparation and preparation method thereof
A freeze-dried preparation, the technology of citabine, is applied in freeze-dried delivery, pharmaceutical formulations, medical preparations with inactive ingredients, etc. High safety and good batch reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Prescription 1
[0071] Decitabine
50g
Potassium dihydrogen phosphate
68g
11.6g
5000ml
[0072] Add water for injection to
10000ml
production
1000 bottles
[0073] Configuration and lyophilization steps:
[0074] Weigh the prescribed amount of potassium dihydrogen phosphate and sodium hydroxide, add an appropriate amount of water for injection, and stir to dissolve. Add the prescribed amount of ethanol, add water for injection to the prescribed amount, and stir well. Add activated carbon for needles (about 0.2%) to the above mixed solution, stir at room temperature for 15-30 minutes, filter and decarbonize to obtain an excipient solution, put it in an ice-water bath for 15-60 minutes (about 5°C), and set aside. Weigh the prescribed amount of decitabine, add it to the excipient solution, and dissolve it quickly by ultrasonication and stirring under i...
Embodiment 2
[0090] Prescription 2
[0091] Decitabine
50g
Potassium dihydrogen phosphate
68g
11.6g
5000ml
Add water for injection to
10000ml
production
1000 bottles
[0092] Weigh the prescribed amount of potassium dihydrogen phosphate and sodium hydroxide, add an appropriate amount of water for injection, and stir to dissolve. Add the prescribed amount of methanol, add water for injection to the prescribed amount, and stir evenly. Add activated carbon for needles (about 0.2%) to the above mixed solution, stir at room temperature for 15-30 minutes, filter and decarbonize to obtain an excipient solution, put it in an ice-water bath for 15-60 minutes (about 5°C), and set aside. Weigh the prescribed amount of decitabine, add it to the excipient solution, and dissolve it quickly by ultrasonication and stirring under ice-water bath conditions. Add activated carbon for needles (abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com