Composition of oral pharmacologic active agent
A technology of physiological saline and uniform mixing, which can be used in drug combinations, pharmaceutical formulations, metabolic diseases, etc., can solve the problems of complex preparation process and difficult industrialized production, and achieve improved bioavailability, improved permeability, and improved oral bioavailability. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of oral liquid
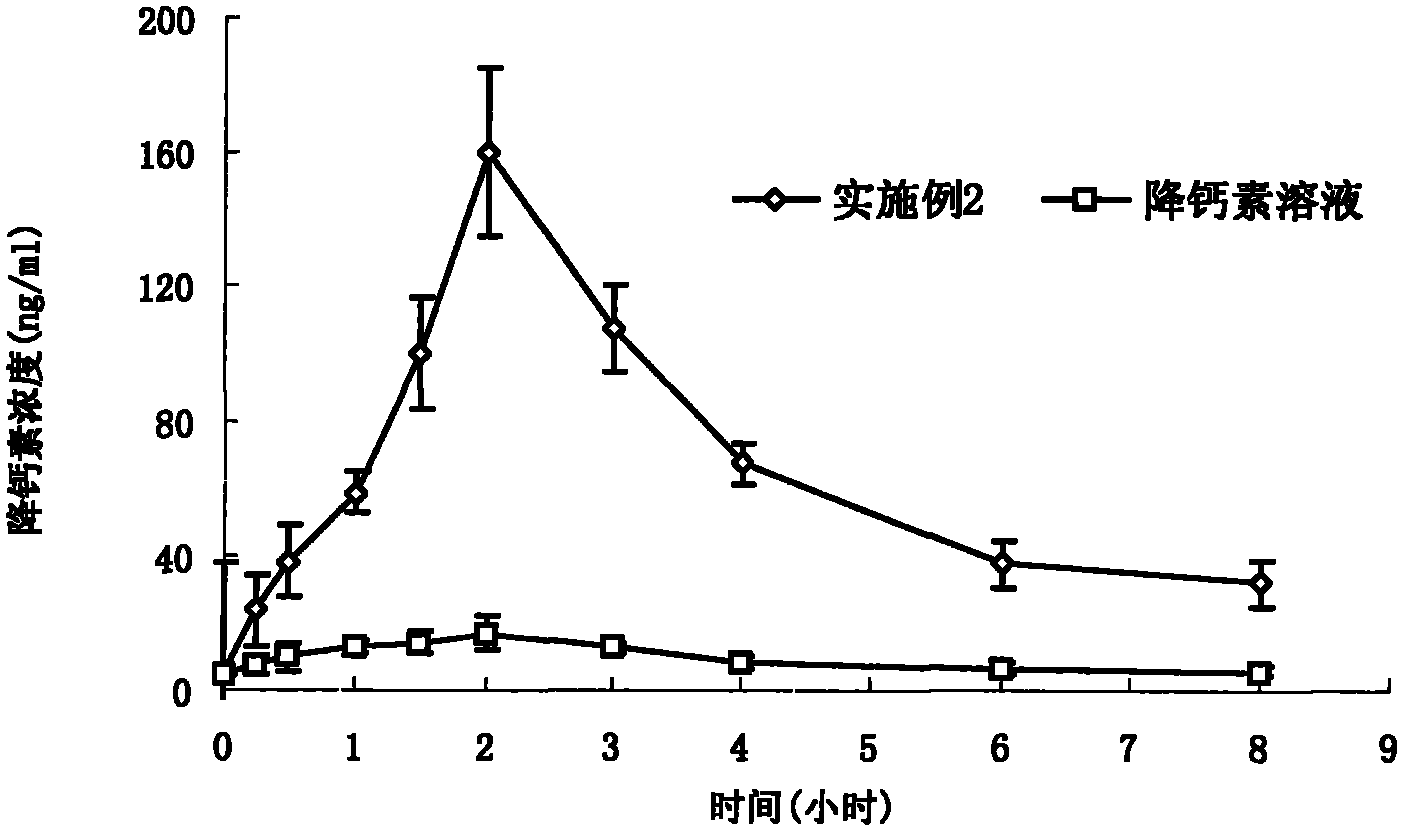
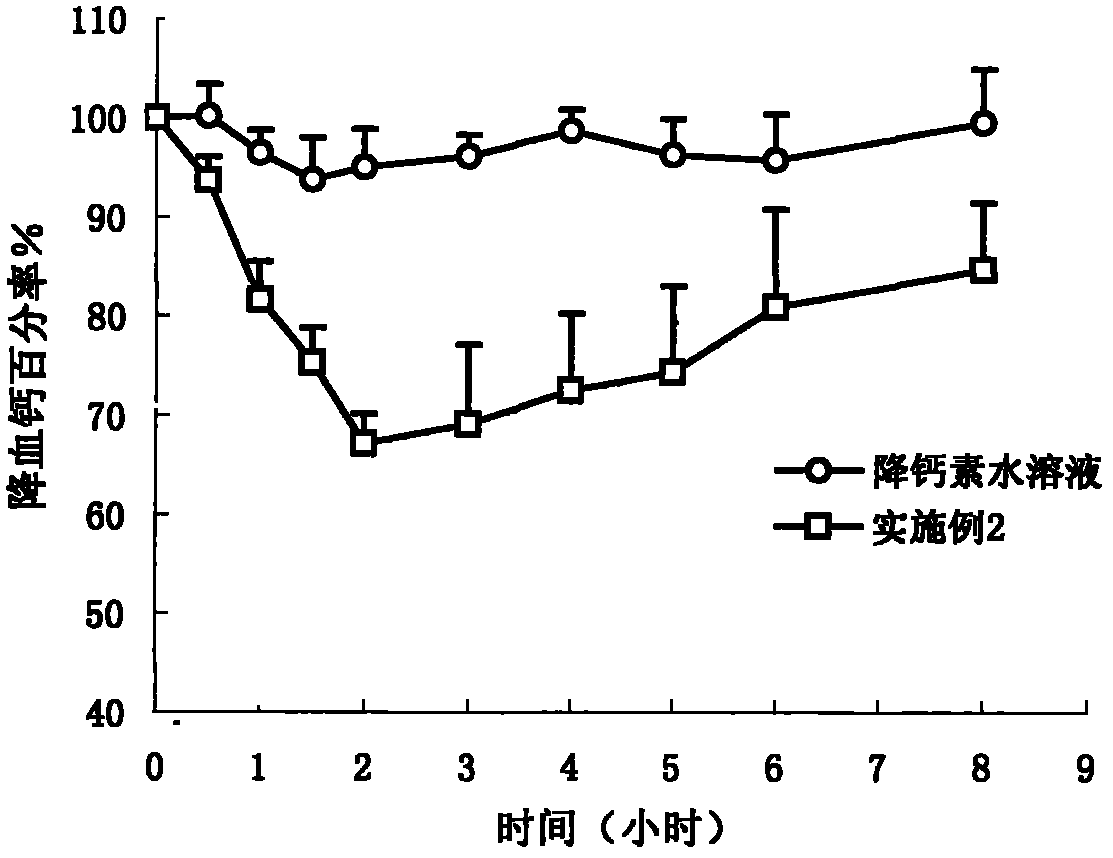
[0039]Dissolve calcitonin 0.2mg and edetate disodium 1.0mg in 0.5ml normal saline, mix Tween801.0mg, Span801.2mg, soybean oil 1.4mg, ethanol 1.1mg and other components evenly, add appropriate amount of Bath Sweet and benzalkonium bromide, mix evenly, obtain 5ml clear mixed solution, then fill it in the vial, add a cap, seal, and radiate sterilization to get final product.
Embodiment 2
[0040] Embodiment 2: the preparation of soft capsule
[0041] Dissolve calcitonin 20mg and salicylic acid 10mg in 45ml of normal saline, mix and dissolve Tween80 91mg, soybean lecithin 91mg, medium chain oil 182mg, propylene glycol 91mg and other components evenly, and add appropriate amount of vitamin E and benzalkonium chloride , mixed evenly and compressed into 1000 soft capsules.
Embodiment 3
[0042] Embodiment 3: the preparation of soft capsule
[0043] Dissolve 19mg of low-molecular-weight heparin and 1.6mg of sodium deoxycholate in 48ml of normal saline, mix and dissolve 76mg of Tween80, 76mg of soybean lecithin, 228mg of caprylic / capric glyceride, 76mg of propylene glycol and other components, and add an appropriate amount of vitamin E, benzalkonium chloride, after mixing uniformly, be compressed into 1000 soft capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com