Application of sulfated flavonoid glycoside in preparation of herpes simplex virus resistant drug
A herpes simplex virus and drug technology, applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of unseen and weak anti-HIV activity
Inactive Publication Date: 2011-06-15
SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
View PDF1 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
It has been reported in the literature that this compound has weak anti-HIV activity (RowleyDavidC, HansenMarkST, RhodesDenise, et al, Bioorganic Medicinal Chemistry, 2002, 10: 3619-3625.), and has the antioxidant function of repairing the skin damaged by ultraviolet light irradiation (Regalado ErikL, Rodríguez María, MenéndezRoberto, etal, MarBiotechnol, 2009, 11:74-80.), but no report on the anti-herpes simplex virus activity of thalassiolinB
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
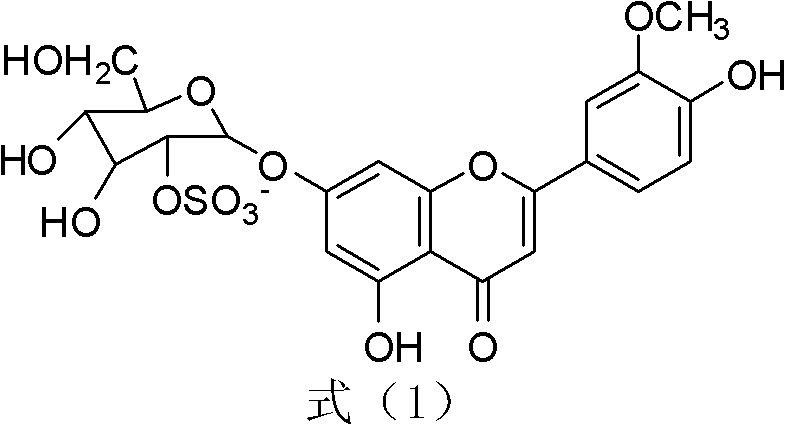
The invention relates to novel application of chrysoeriol-7-beta-D-glucose-2''-sulfate shown in a formula (1) in preparation of herpes simplex virus resistant drug. The chrysoeriol-7-beta-D-glucose-2''-sulfate has no remarkable toxicity on a host Vero cell, the maximum toxicity-free concentration (TC0) of the chrysoeriol-7-beta-D-glucose-2''-sulfate to the Vero cell is more than 260mu g / mL, the inhibiting concentration (IC50) of the chrysoeriol-7-beta-D-glucose-2''-sulfate to HSV-1 (Herpes Simplex Virus) under a combined action manner is 3.772mu g / mL, the IC50 of the chrysoeriol-7-beta-D-glucose-2''-sulfate to the HSV-1 under a direct virus inactivating action manner is 0.084mu g / mL, the IC50 of the chrysoeriol-7-beta-D-glucose-2''-sulfate to the HSV-1 under an adsorption inhibiting action manner is 14.5mu g / mL, and the IC50 of the chrysoeriol-7-beta-D-glucose-2''-sulfate to the HSV-1 under a transfer inhibiting action manner is 12.06mu g / mL. The chrysoeriol-7-beta-D-glucose-2''-sulfate ensures that the virus cannot normally adsorb and penetrate in the cell through direct virus inactivation on the basis of preliminary guess, thereby blocking the HSV-1 infection outside the cell.
Description
Application of a kind of sulfated flavone glycoside in the preparation of anti-herpes simplex virus medicine technical field The present invention relates to a kind of sulfated flavonoid glycosides, specifically aureothin-7-β-D-glucose-2″-sulfate (chrysoeriol7-β-D-glucopyranosyl) represented by the following formula (1) -2 "-sulphate, also known as Thalassiolin B) in the preparation of new applications of anti-herpes simplex virus drugs. Background technique Human herpes simplex virus type 1 (Herpessimplex1virus, HSV-1) can cause a variety of diseases such as cold sores, herpetic keratoconjunctivitis and neonatal encephalitis, and a higher proportion of immunocompromised patients such as AIDS, cancer patients and organ transplants are infected HSV-1 virus. Scarring caused by HSV-1 infection is a leading cause of blindness in developed countries. At present, the drugs commonly used clinically for the treatment of HSV-1 are mainly nucleosides such as acyclovir (ACV), and i...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/7048A61P31/22
Inventor 漆淑华
Owner SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com