Dasatinib polymorphic substance as well as preparation method and medicinal composition thereof
A technology of polymorphs and dasatinib, applied in the field of polymorphs of dasatinib, preparation methods of polymorphs and pharmaceutical compositions thereof, can solve problems such as reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
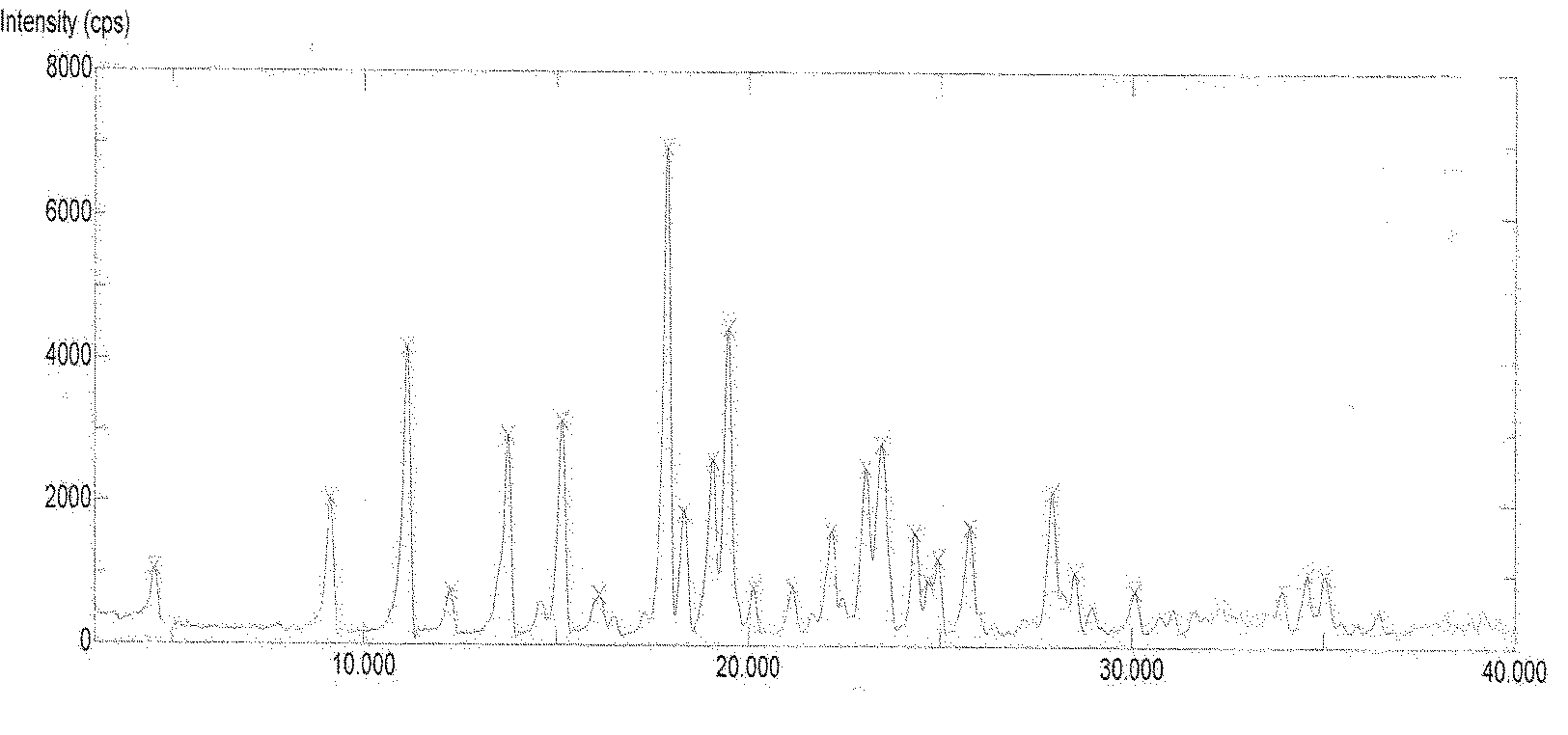
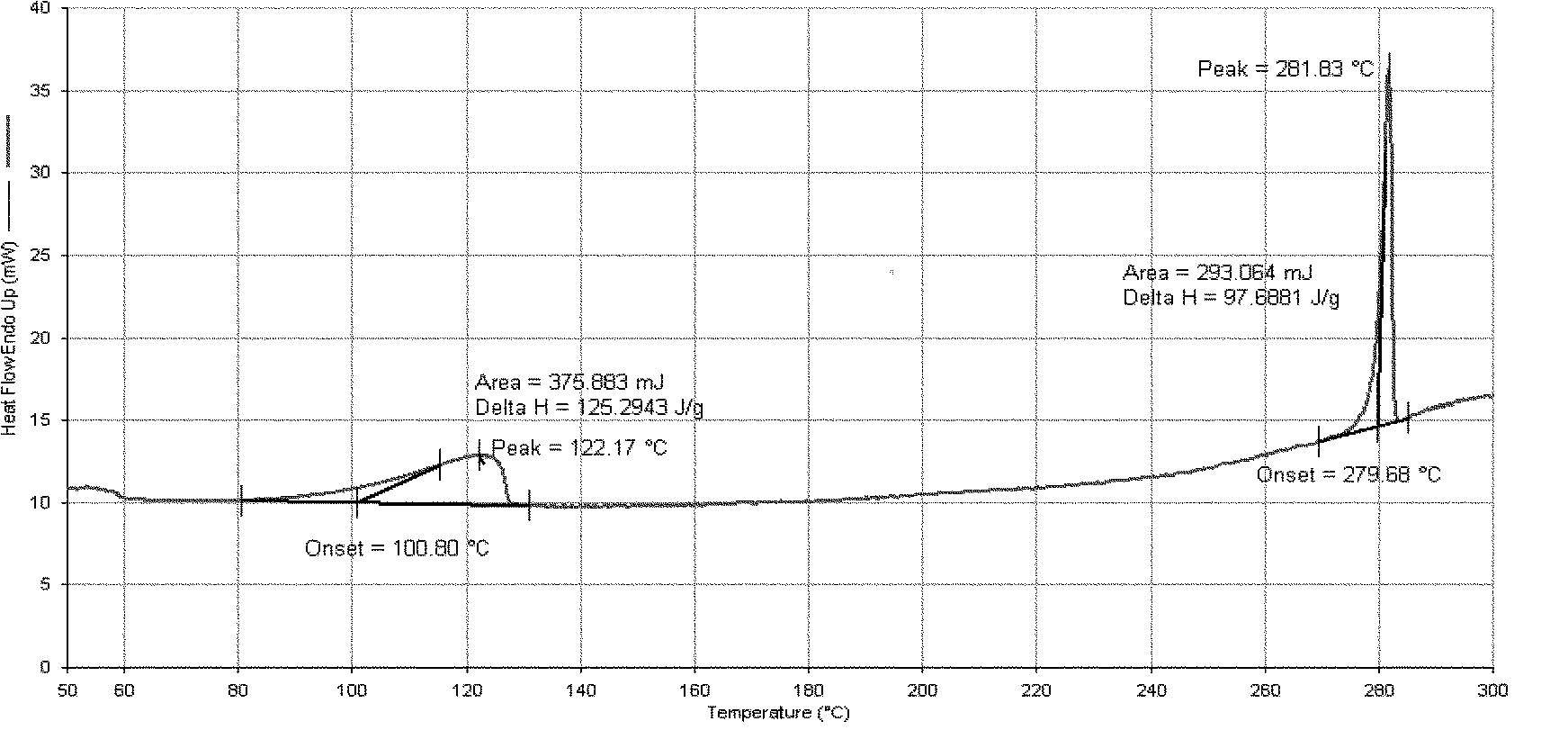
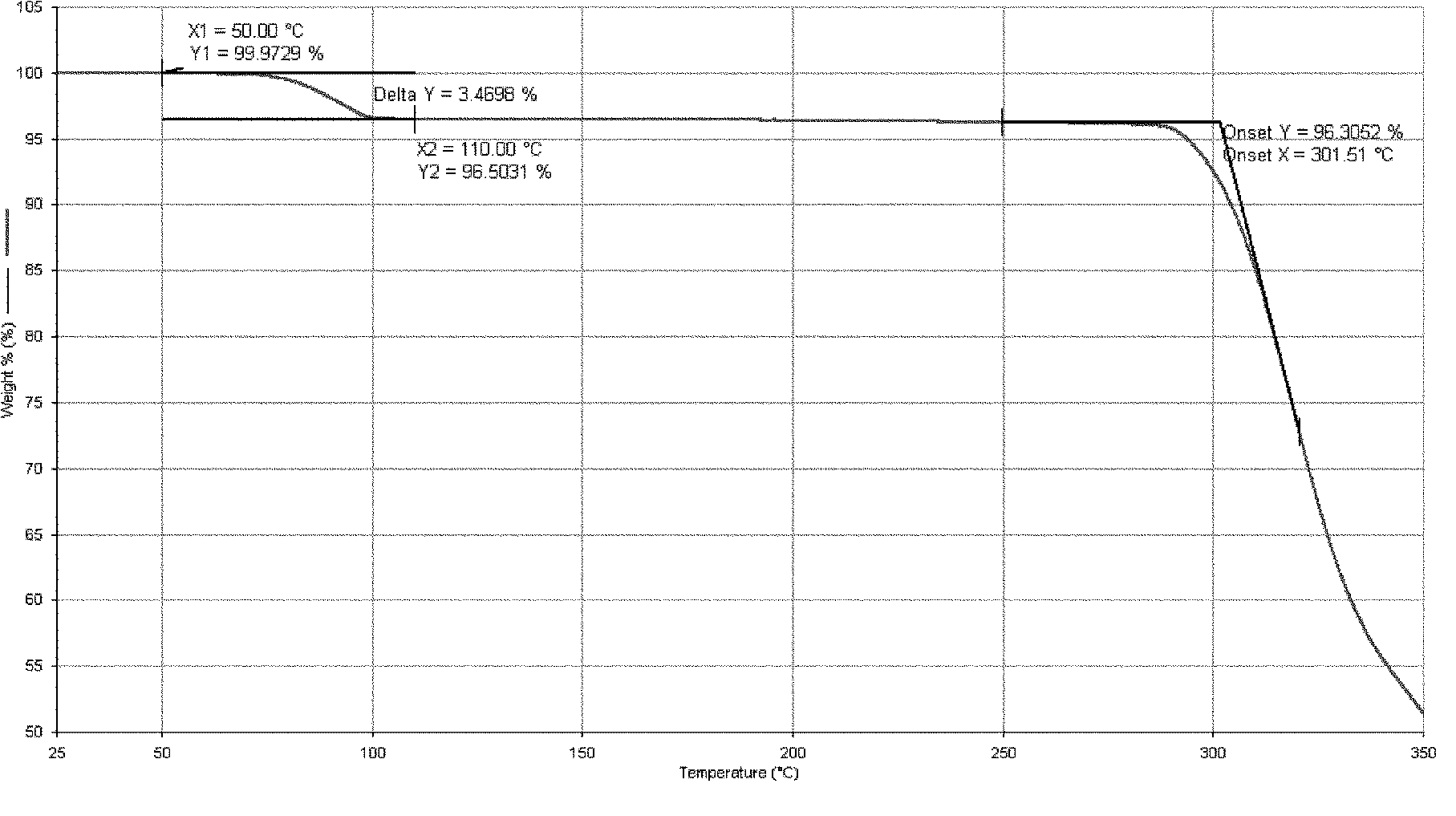
[0095] Add 16 g of Dasatinib and 160 ml of purified water into the reaction flask, heat up to reflux with stirring, cultivate the crystals for 3 hours, and then naturally lower to room temperature and filter with suction. The filter cake is rinsed with an appropriate amount of water and drained. The filter cake was dried under reduced pressure (-0.095MPa) at about 50°C, and dried with phosphorus pentoxide. 15.4 g of white solid was obtained. Yield: 96.3%.
[0096]
Embodiment 2
[0098] Add 10 g of Dasatinib and 90 ml of purified water into the reaction flask, heat up to reflux with stirring, cultivate the crystals for 4 hours, reduce to room temperature, and filter with suction. The filter cake is rinsed with an appropriate amount of water and drained. The filter cake was dried under reduced pressure (-0.095MPa) at about 50°C, and dried with phosphorus pentoxide. 9.8 g of white solid was obtained. Yield: 98.0%.
[0099]
Embodiment 3
[0101] The prescription and preparation process of Dasatinib capsules:
[0102] The polymorph III of dasatinib was prepared into a capsule preparation containing 50 mg with several excipients according to the following method.
[0103]
[0104] The manufacturing method of capsules containing dasatinib polymorph III is to add the first four of the above excipients and dasatinib polymorph III with water to prepare a soft material, and the soft material is made into a wet material. After the granules are dried, the dried granules are uniformly mixed with magnesium stearate and then filled into the capsule shell to obtain dasatinib capsules.
[0105] Dissolution curve:
[0106] Capsule formula 1
[0107] time
[0108] See the dissolution profile of capsule formula 1 Figure 5 .
[0109] Capsule formula 2
[0110] time
[0111] See the dissolution profile of capsule formula 2 Image 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com