Japanese blood fluke protein and application thereof
A protein and schistosome technology, applied in the field of Schistosoma japonicum protein, to achieve the effect of reducing the number of worms and good immune prevention effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Expression and purification of embodiment 1 rSjGALE recombinant protein
[0027] The coding sequence (SEQ ID NO: 2) of Schistosoma japonicum UDP-glucose-4-epimerase gene (SjGALE) was inserted into the polyclonal restriction site of prokaryotic expression vector pET28a(+) to construct pET28a-SjGALE recombinant Expression vector, after the recombinant expression vector is identified correctly by sequencing, the following expression and purification steps are carried out.
[0028] 1. Prokaryotic expression of recombinant proteins
[0029] 1) Take a single clone colony containing the recombinant plasmid and inoculate it in LB culture medium containing the corresponding antibiotic, and cultivate overnight at 37°C with shaking.
[0030] 2) Culture with shaking at 37°C until OD600 reaches 0.4-0.6, and add IPTG to a final concentration of 1 mM to induce expression.
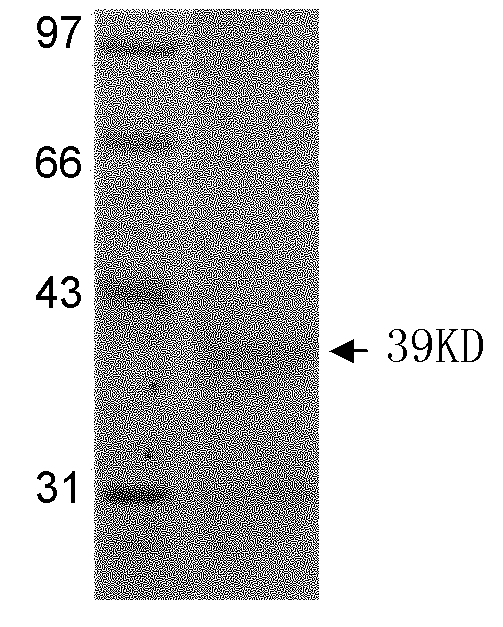
[0031] 3) Collect the bacterial fluids before and after induction respectively, and analyze and verify by SDS-...
Embodiment 2
[0041] The immunogenicity detection of embodiment 2 recombinant protein
[0042] 1) Run SDS-PAGE electrophoresis on the expressed and purified recombinant protein.
[0043] 2) Cut the nitrocellulose membrane and six-layer filter paper of the same size as the glue block, and put the nitrocellulose membrane and filter paper into the buffer solution for soaking; place three layers of filter paper, gel, nitrocellulose membrane, Three layers of filter paper; fill the tank with transfer buffer and ice pack, 280mA, transfer for 2h;
[0044] 3) After the electrophoretic transfer, put the transferred nitrocellulose membrane into 3% bovine serum albumin and block overnight at 4°C;
[0045] 4) Discard the blocking solution, wash three times with TBST, each time for 10 minutes;
[0046] 5) Add 1:200 diluted anti-Schistosoma japonicum adult soluble antigen antibody (preserved in the laboratory) as the primary antibody, and incubate at room temperature for 1 hour;
[0047] 6) Wash three ...
Embodiment 3
[0052] Embodiment 3 Immunoprotective experiment
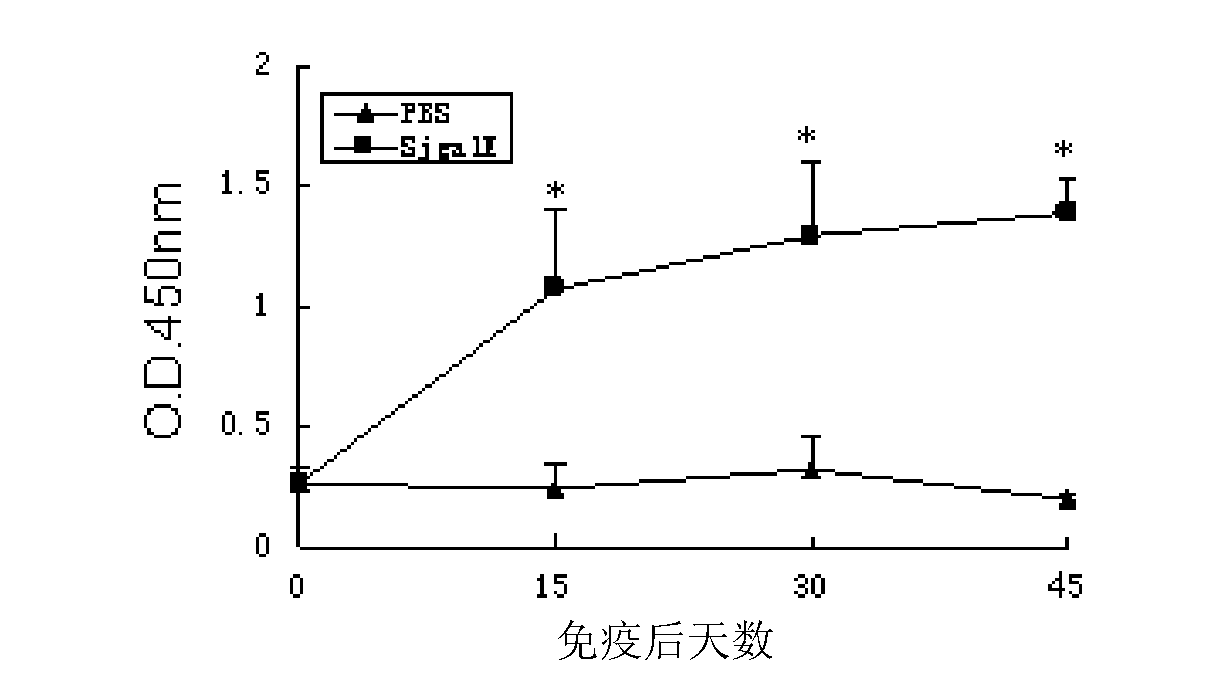
[0053] 1. Animal immunization experiments
[0054] 1) BalB / C mice were randomly divided into 2 groups, 10 in each group.
[0055] 2) The immune protection test was carried out with the recombinant protein rSjGALE of the partial amino acid sequence of Schistosoma japonicum SjGALE (SEQ ID NO: 1), and an adjuvant control group was set at the same time.
[0056] 3) In the experiment, each mouse was injected with a mixture of 50 μg of recombinant protein and an equal volume of Freund's complete / incomplete adjuvant, and the adjuvant control group was injected with a mixture of PBS and adjuvant.
[0057] Immunization was performed once every two weeks, three times in total, blood was collected from orbital veins before and two weeks after each immunization, and serum was separated and stored at -20°C.
[0058] 4) Two weeks after the third immunization, the abdominal skin patch of 30 cercariae per mouse was used to challenge the infect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com