Method for preparing phenylaniline
A technology of aminobiphenyl and basebiphenyl, which is applied in the field of preparation of aminobiphenyl, and can solve problems such as increased cost, difficulty in cleaning, and environmental pollution by iron powder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The invention provides a preparation method of aminobiphenyl, comprising:
[0050] a) The benzonitrile shown in formula II, the phenylboronic acid shown in formula III, the first basic compound and the nickel catalyst are mixed in the first solvent to carry out the Suzuki coupling reaction to obtain the cyanobiphenyl shown in formula IV ;
[0051] b) mixing the cyanobiphenyl with a second basic compound and hydrogen peroxide in a second solvent, and hydrolyzing the cyanobiphenyl to obtain amidobiphenyl shown in formula V;
[0052] c) mixing the amidobiphenyl with the third basic compound and sodium hypohalite in a third solvent to undergo a Hofmann degradation reaction to obtain the aminobiphenyl represented by formula I.
[0053]
[0054]
[0055] Among them, R 1 is chlorine, bromine, iodine, sulfonate, carbonate or alkyl ester; R 2 is alkyl, alkoxy, cyano, amido or hydrogen; R 3 is chlorine, bromine or iodine; R 4 is alkyl, alkoxy, cyano, amido or hydrogen. ...
Embodiment 1
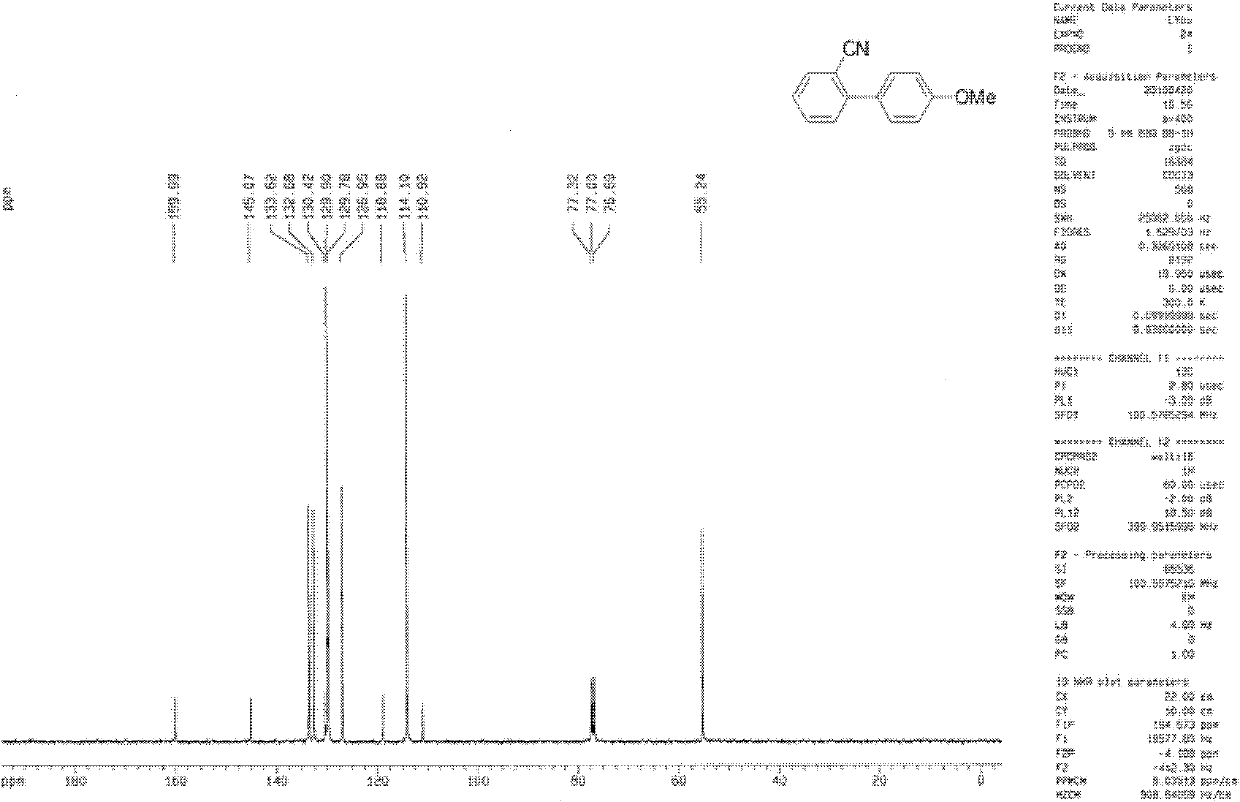
[0083] Under a nitrogen atmosphere, mix 0.7819g (5mmol) of 4-chlorophenylboronic acid, 1.0318g (7.5mmol) of o-chlorobenzonitrile, 3.1840g (15mmol) of anhydrous potassium phosphate, 0.0054g (0.01mmol) of nickel chloride, and Add 1,3-bis(diphenylphosphine)propane at an equimolar ratio of nickel to the reactor, add 20 g of dioxane to the reactor, react at 100°C for 6 h under a nitrogen atmosphere, and quench with saturated brine. The reaction was quenched, extracted with dichloromethane, dried, separated on silica gel, and the dichloromethane was evaporated to obtain 1.0149 g of 4'-chloro-2-cyanobiphenyl with a yield of 82.8%. The hydrogen spectrum and the carbon spectrum of the 4'-chloro-2-cyanobiphenyl prepared in embodiment 1 are:
[0084] 1 H NMR (CDCl 3 , 600MHz) δ: 7.26(m, 6H), 7.64(t, J=7.5Hz, 1H), 7.77(d, J=7.7Hz, 1H)); 13 C NMR (CDCl 3 , 150MHz) δ: 111.3, 118.4, 127.9, 129.0 (2C), 129.9, 130.1 (2C), 132.9, 133.8, 135.1, 136.5, 144.2;
Embodiment 2
[0086] Under a nitrogen atmosphere, mix 1.5637g (10mmol) of 4-chlorophenylboronic acid, 1.3757g (10mmol) of o-chlorobenzonitrile, 6.0681g (30mmol) of anhydrous potassium phosphate, 0.0108g (0.02mmol) of nickel chloride and Add 1,3-bis(diphenylphosphine)propane with an equimolar ratio of nickel into the reactor, add 40g of dioxane into the reactor, react at 100°C for 8h under a nitrogen atmosphere, and quench with saturated saline Reaction, extraction with dichloromethane, drying, separation on silica gel, evaporation of dichloromethane to obtain 2.0200 g of 4'-chloro-2-cyanobiphenyl with a yield of 82%. The hydrogen spectrum and the carbon spectrum of the 4'-chloro-2-cyanobiphenyl prepared in embodiment 2 are:
[0087] 1 H NMR (CDCl 3 , 600MHz) δ: 7.26(m, 6H), 7.64(t, J=7.5Hz, 1H), 7.77(d, J=7.7Hz, 1H)); 13 C NMR (CDCl 3 , 150MHz) δ: 111.3, 118.4, 127.9, 129.0 (2C), 129.9, 130.1 (2C), 132.9, 133.8, 135.1, 136.5, 144.2;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com