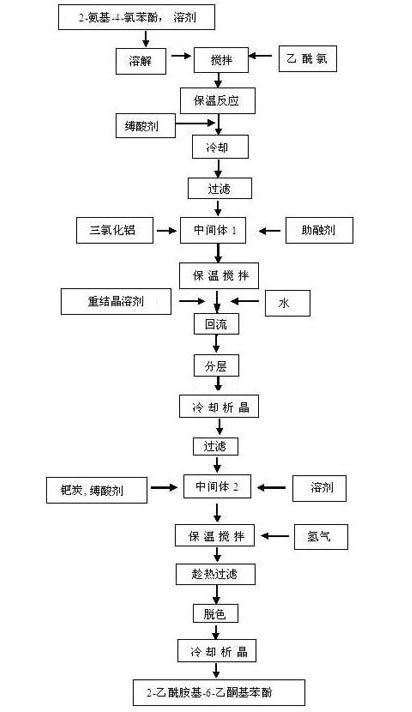
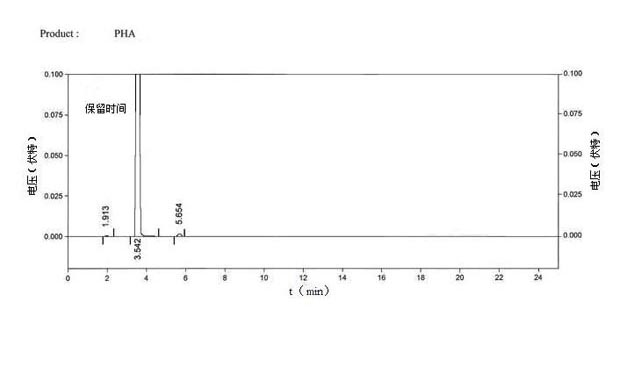
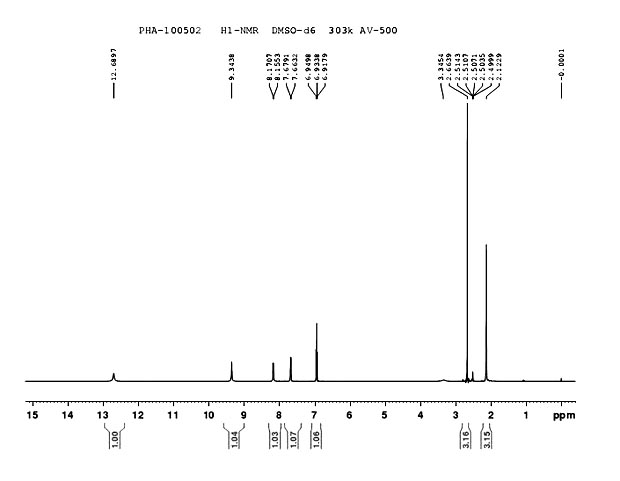
Method for preparing 2-acetamido-6-acetylphenol
An acetone-based phenol and acetamido-based technology is applied in the field of preparing 2-acetamido-6-ethanone-based phenol, can solve the problems of high cost, large pollution, long route and the like, and achieves low cost, low pollution, The effect of a wide range of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1, preparation of intermediate I: (2-acetamido-4-chloro)phenyl acetate
[0023] Add 24.0g of 2-amino-4-chlorophenol (0.168mol) and 88.0g of ethyl acetate into a 250mL three-necked flask, slowly add 15.1g of acetyl chloride (0.420mol) dropwise in a water bath, and drop it for about 1 hour. Then, the temperature was raised to 70° C. and kept under reflux for 2 hours, and then 21.0 g of triethylamine (0.208 mol) was slowly added dropwise. At this time, the pH of the solution was neutral. After about 0.5 hours of dropping, the mixture was continued to keep stirring for 15 minutes. TLC detects that the raw material point disappears, and there is only one point of the product. The reaction solution was cooled to 10°C and then filtered. The filtrate can be applied directly for the next use. Add the filter cake and 120mL water into a 250L reaction flask, stir at 40°C for 1 hour, then filter, wash the filter cake 3 times with water (5mL*3), and then dry the product to obt...
Embodiment 2
[0042] Embodiment 2 Pilot test scheme
[0043] Step 1, preparation of intermediate I: (2-acetamido-4-chloro)phenyl acetate
[0044] Add 24.0kg of 2-amino-4-chlorophenol (0.168kmol), 34.0kg of triethylamine (0.336kmol) and 88kg of ethyl acetate into a 200L reactor, and slowly add 15.1kg of acetyl chloride (0.420kmol) dropwise at room temperature , and then heated to 70 ° C to maintain a slight reflux reaction for 4h. The reaction solution was cooled to 10°C and then centrifuged. The filtrate can be applied directly for the next use. Add the filter cake and 120kg of water into a 200L reactor, stir at 40°C for 1 hour, centrifuge, wash the filter cake with water 3 times (5L*3), and then dry the product to obtain 34.2kg of the product, with a yield of 90%.
[0045] Step 2, preparation of intermediate II: 2-acetamido-4-chloro-6-acetonylphenol
[0046]Intermediate I (0.079kmol) and 32.0kg AlCl prepared by 18.0kg step one 3 (0.240kmol), 14.7kg NaCl (0.252kmol) were added to a 200...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com