Immunogenic composition
A technology of immunogenicity and composition, which can be used in drug combinations, antibody medical components, antibacterial drugs, etc., and can solve problems such as inapplicability to humans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
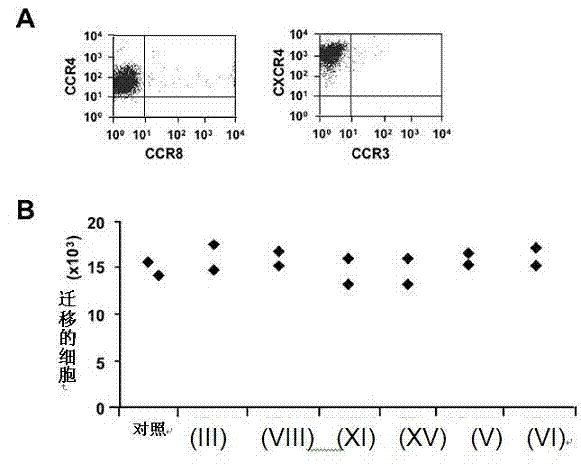
[0115] Evaluation of CCR4 Antagonism and Specificity by Chemotaxis Assay
[0116] 116 CCR4 antagonists tested for inhibition of CCR4 + Ability of CCL22-regulated chemotaxis of human Caucasian acute T lymphoblastoid leukemia cell line CCRF-CEM ( figure 2 A). 16 compounds (about 13.7%) inhibited the migration of CCRF-CEM cells regulated by CCR4, IC 50 value (concentration required to inhibit migration by 50%) at 179 x 10 -11 to 229 x 10 -14 within the M range.
[0117] CCRF-CEM can also express another chemokine receptor, CXCR4 ( figure 2 A), which allows the specificity of CCR4 antagonists to be tested. With the exception of one antagonist, compounds had no effect on CXCR4-regulated migration ( figure 2 B) or cell viability (data not shown) had no effect, even at concentrations above their IC 50 value (approximately 2 ) 1000 times.
Embodiment 2
[0119] Disruption of human Treg recruitment regulated by CCL22 and CCL17 by CCR4 antagonists
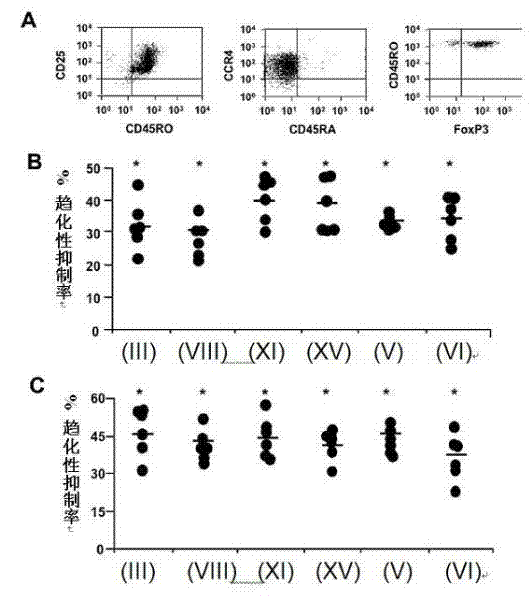
[0120] Treg negatively regulates immune responses induced by professional antigen-presenting cells. Therefore, inhibiting the CCR4-dependent recruitment of Tregs regulated by CCL22 and CCL17 represents a potential target for promoting immune responses. CD4 expressing high levels of CD25 + CD45RO + Tregs enriched in T cells were isolated from peripheral blood mononuclear cells (PBMC) of healthy donors. These CD4+CD25 高 cells expressed FoxP3 and CCR4 ( image 3 A). In addition, they failed to proliferate and secrete T cell cytokines after in vitro stimulation, and also inhibited the proliferation of co-cultured conventional T cells (data not shown), so it can be confirmed that the isolated CD4+CD25 高 Cells are bona fide Tregs.
[0121] Six compounds (compounds of formula (III), (V), (VI), (VIII), (XI) and (XV)) were tested for their ability to block Treg migration regulated b...
Embodiment 3
[0123] Inhibition of chemotaxis of human Th2 cells regulated by CCL22 and CCL17 by CCR4 antagonists
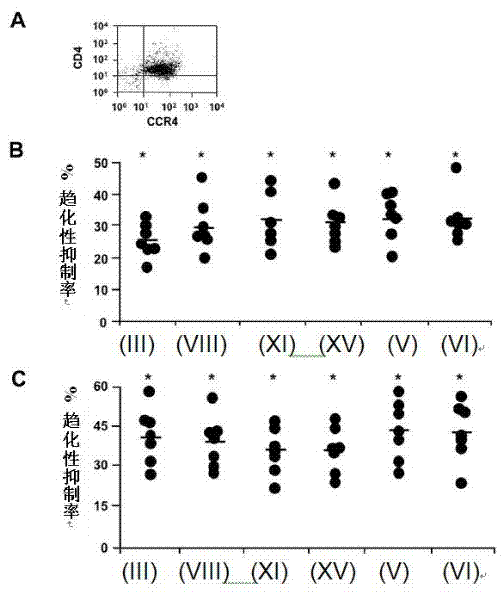
[0124] Polarized effector T cells are known to influence the development of immune responses. Th2-biased responses can suppress Th1-biased cellular immune responses, which are thought to be more protective against intracellular pathogens (Szabo SJ et al. (2003) Annu Rev Immunol 21:713-758). In addition to Treg, polarized human Th2 cells express CCR4 and migrate in response to CCR4 ligands (Bonecchi R, et al. (1998) J Exp Med 187:129-134). Therefore, it is important to determine whether novel adjuvants can inhibit the migration of polarized Th2 cells, as these cells may be harmful or useful depending on the target pathogen. Figure 4 A demonstrates that polarized Th2 cells generated in vitro can express CCR4. Further, all six CCR4 antagonists significantly inhibited CCL22- and CCL17-directed migration of Th2 cells when observed with Treg ( Figure 4 B, C), and have a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com