Compound sodium aescinate cataplasm
A technology of compound aescin and sodium aescinate, which is applied in the directions of pharmaceutical formula, flake delivery, organic active ingredients, etc., can solve the problems that there is no compound escinate sodium cataplasm, and improve the therapeutic effect and load The effect of large dose and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
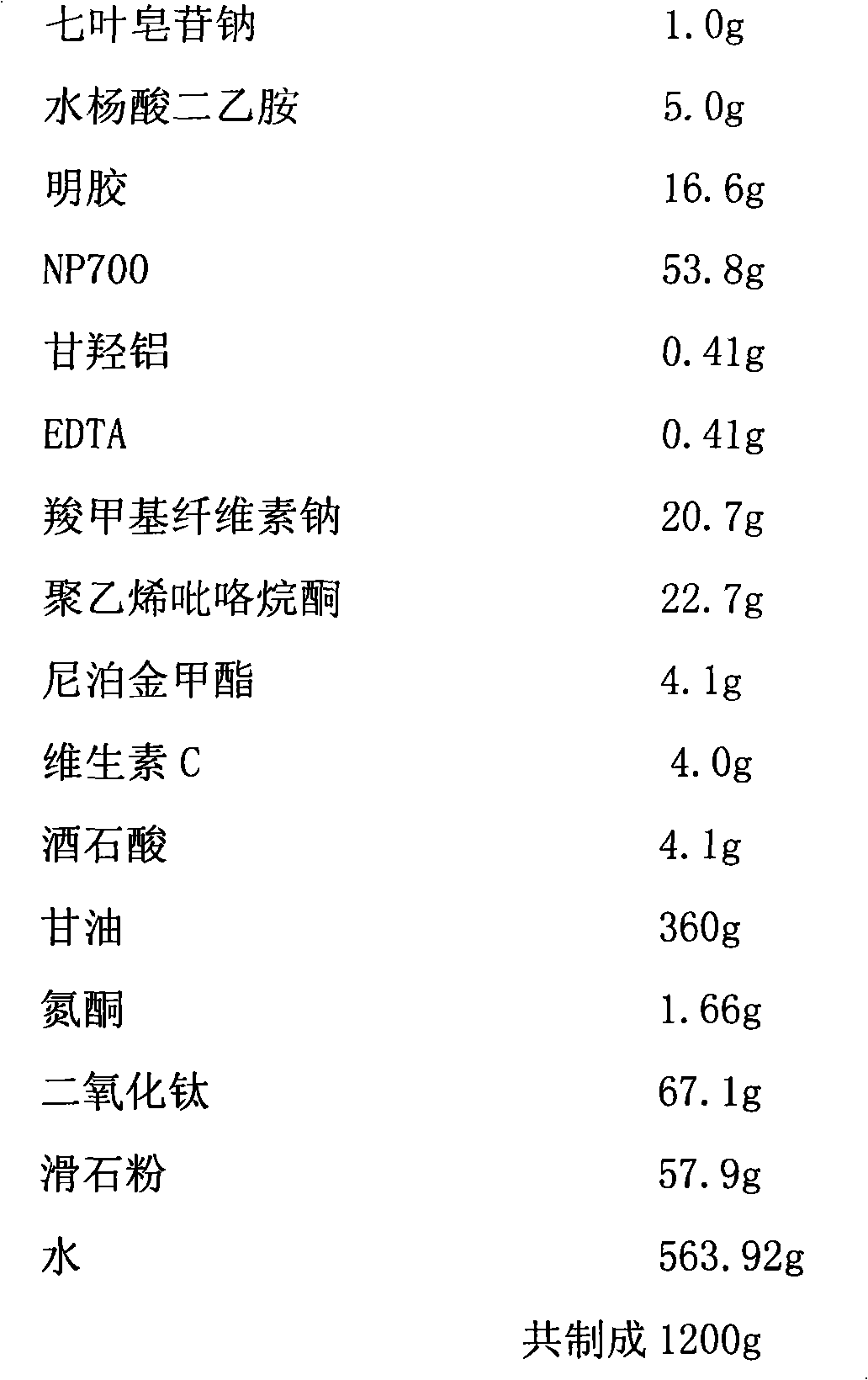
[0051] Compound sodium aescinate cataplasm, main ingredient content (0.5%)
[0052] prescription:
[0053]
[0054] Preparation:
[0055] a. Dissolve methylparaben and vitamin C in azone, then disperse aluminum glyoxate and EDTA through a 200-mesh sieve in azone, then add the mixture of ground titanium dioxide, talcum powder and glycerin, get the oily phase;
[0056] b. Physically mix NP700, sodium carboxymethylcellulose, and polyvinylpyrrolidone, and disperse them into the oil phase of step a;
[0057] c. tartaric acid is dissolved in 240 grams of water and becomes a solution for subsequent use;
[0058] d. Dissolve gelatin in water at 1:10, swell for 15 minutes at 40°C, dissolve the main ingredients (sodium aescinate and diethylamine salicylate) with the remaining water, and then put the gelatin and the main ingredients at 30°C Add to the oil phase of step b under the condition, then add the solution of step c in 6 times, and stir for 30 minutes to obtain the paste; ...
Embodiment 2
[0061] Preparation of compound sodium aescinate cataplasm, main ingredient content (1%)
[0062] prescription:
[0063]
[0064] Preparation:
[0065] a. Dissolve methylparaben in azone and propylene glycol, then disperse aluminum glyoxate and EDTA-2Na through a 200 mesh sieve, then add the mixture of ground titanium dioxide, zinc oxide and glycerin to obtain oil phase;
[0066] b. Physically mix sodium polyacrylate, sodium carboxymethyl cellulose, and gum tragacanth, and disperse them in the oil phase of step a;
[0067] c. tartaric acid is dissolved in 240 grams of water and becomes a solution for subsequent use;
[0068] d. Dissolve the main ingredients (sodium aescinate and diethylamine salicylate) with the remaining water, then add to the oil phase of step b, then add the solution of step c in 6 times, and stir for 30 minutes to obtain a paste body;
[0069] e. Spread the paste in step d on the non-woven fabric, cover with release paper, cut into 120 pieces, and p...
Embodiment 3
[0071] Preparation of compound sodium aescinate cataplasm, main ingredient content (4%)
[0072] prescription
[0073]
[0074]
[0075] Preparation:
[0076] a. Mix azone and propylene glycol, then disperse aluminum hydroxide and EDTA-2Na passing through a 200-mesh sieve, and then add the mixture of ground kaolin and glycerin to obtain an oil phase;
[0077] b. Physically mix sodium polyacrylate, sodium carboxymethyl cellulose, and gum tragacanth, and disperse them in the oil phase of step a;
[0078] c. citric acid is dissolved in 240 grams of water and becomes a solution for subsequent use;
[0079] d. Dissolve the main ingredients (sodium aescinate and diethylamine salicylate) and sodium bisulfite with the remaining water, then add to the oil phase of step b, then add the solution of step c in 6 times, and stir for 30 Minutes to get the paste;
[0080] e. Spread the paste in step d on the non-woven fabric, cover with release paper, cut into 120 pieces, and pack t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com