Pitavastatin calcium enteric sustained-release micropill preparation and preparation method thereof
A technology of pitavastatin calcium and sustained-release pellets, which is applied in the direction of bulk delivery, metabolic diseases, and active ingredients of heterocyclic compounds, and can solve problems such as poor stability, prone to configuration transformation, and inconsistent synthesis time periods. Achieve the effect of stable configuration, less adverse reactions, and stable blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
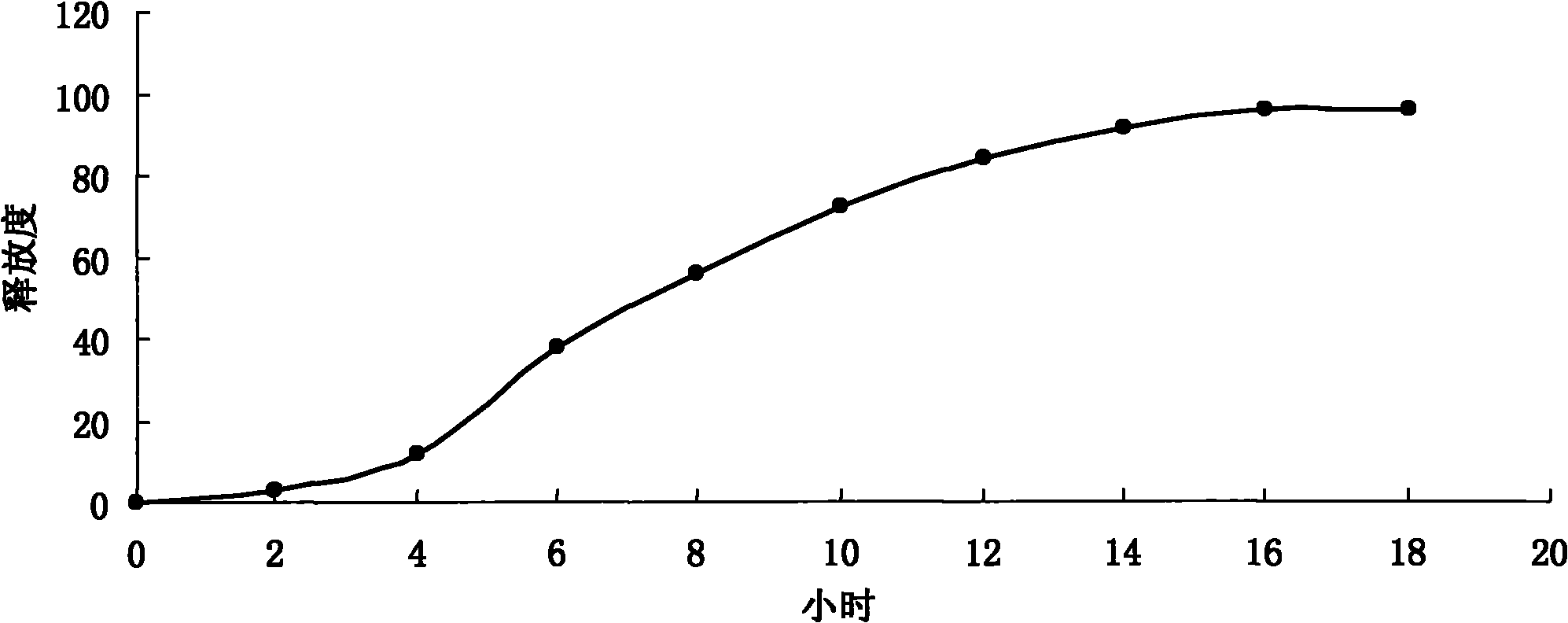
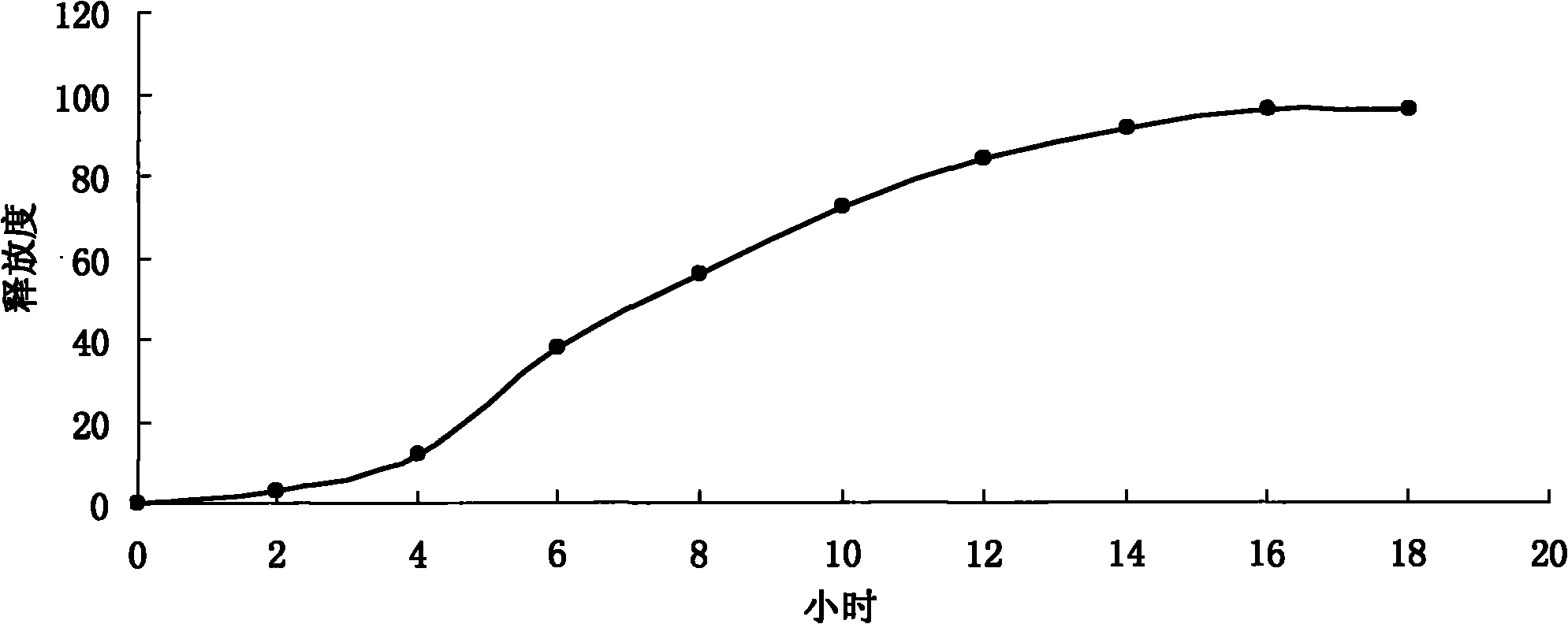
Image
Examples
Embodiment 1
[0041] A pitavastatin calcium enteric-coated sustained-release pellet preparation, the formula of which is as follows:
[0042] Contains Pill Hearts:
[0043] Pitavastatin Calcium 1g
[0044] Starch 30g
[0045] Sodium bicarbonate 20g
[0046] 5% PVP ethanol solution 50g
[0047] (5% PVP ethanol solution is the solution that PVP is dissolved in ethanol and forms, and its weight percentage is 5%);
[0048] Isolation layer:
[0049] Low-substituted hydroxypropyl cellulose 1g
[0050] 2% HPMC ethanol solution 50g
[0051] (2% HPMC ethanol solution is that HPMC is dissolved in ethanol, and its weight percentage is 2%);
[0052] Slow release layer:
[0053] Ethylcellulose 8g
[0054] PVP 2g
[0055] Triethyl citrate 5g
[0056] 95% ethanol (as solvent) 80g
[0057] Enteric layer:
[0058] Eudragit L 24g
[0059] Dibutyl sebacate 2g
[0060] Micronized silica gel 0.5g
[0061] 95% ethanol (as solvent) 100g
[0062] Preparation:
[0063] Take pitavastatin calcium raw ...
Embodiment 2
[0069] Pitavastatin Calcium 2g
[0070] Calcium hydrogen phosphate 15g
[0071] Sucrose 32g
[0072] 60% syrup (weight percentage) 10g
[0073] Sieve granulated sucrose, collect 40-60 mesh sugar cores as seed cores, weigh them and place them in a multi-functional coating granulation pot, use syrup as a binder, sprinkle pitavastatin calcium raw materials and calcium hydrogen phosphate Fine powder, made into pills, dried. Configure each layer of coating solution (coating solution is the isolation layer, slow-release layer and enteric layer), according to the prescription of embodiment 1, the coating solution is wrapped layer by layer on the micro-nine, control spray speed and spray volume, so that Keep the surface of the pellets moist but not sticky, blow hot air to dry, and repeat the operation to obtain controlled-release pellets with a particle size of about 1mm.
Embodiment 3
[0075] Starch blank pellets 100g
[0076] Pitavastatin Calcium 1g
[0077] Calcium citrate 5g
[0078] 2% PVP ethanol solution 120g
[0079] (2% PVP ethanol solution) is that PVP is dissolved in ethanol solution, and its weight percentage is 2%);
[0080] Isolation layer:
[0081] Hypromellose 2.5g
[0082] 95% ethanol solution (as solvent) 50g
[0083] Slow release layer:
[0084] Acrylic resin NE30D 18g
[0085] PEG1500 6g
[0087] water 100g
[0088] Enteric layer:
[0089] Polyacrylic resin Eudragit L 45g
[0090] Dimethyl phthalate 2g
[0092] 95% ethanol (as solvent) 120g
[0093] Preparation process: place 18-24 mesh blank pellets in a fluidized bed, dissolve pitavastatin calcium and calcium citrate in PVP ethanol solution and spray them into the fluidized bed. Then dissolve the hypromellose in 95% ethanol and spray it into the fluidized bed to cover the isolation layer. Mix acrylic resin NE30D and water, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com