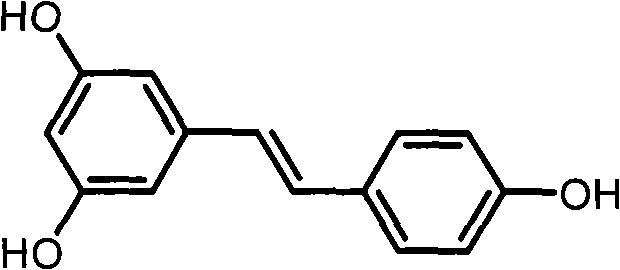
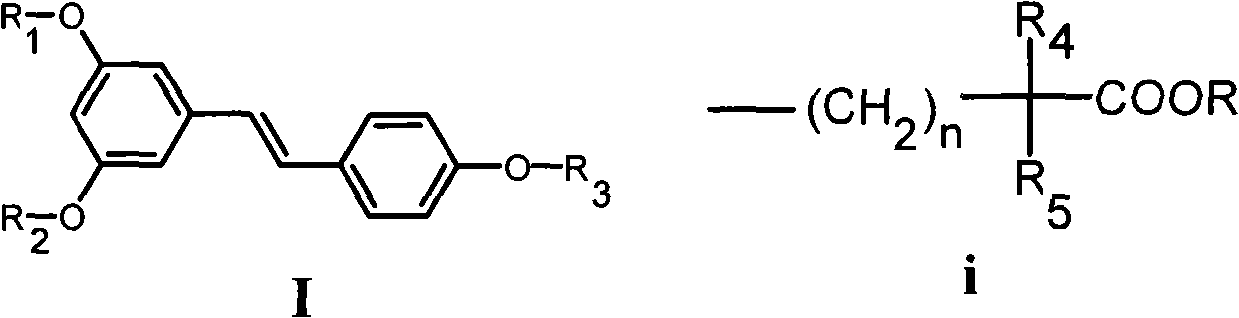
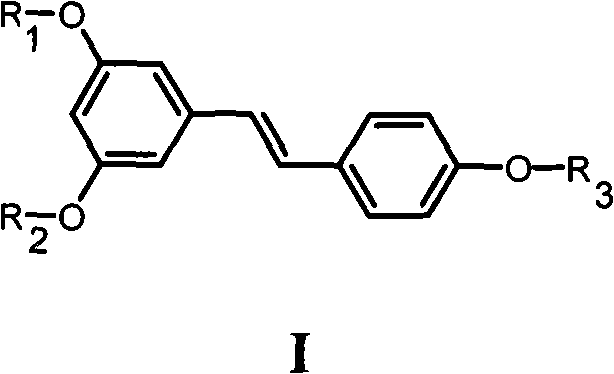
Resveratrol derivative and application thereof to medicament
A pharmacy and compound technology, applied in the field of resveratrol derivatives and their medicinal uses, can solve the problems of the need to improve biological activity, low oral bioavailability, poor water solubility of resveratrol and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1 3,5-dihydroxy-4'-(4-methyl-4-isobutoxycarbonylpentyloxy)-(E)-stilbene (Ia 1 )Synthesis
[0158] Add 10g (43.8mmol) resveratrol, 36.26g (262.8mmol) K 2 CO 3 , 100mL of dry DMF, stirred and heated to 55°C under the protection of nitrogen, added dropwise 38.67g (175.2mmol) of 5-chloro-2,2-dimethylpentanoic acid isobutyl ester, after the addition was completed, continue to heat and react at 55°C for 24h , stop heating, after cooling, add 100mL ethyl acetate to the reaction solution; Wash the resulting solution 3 times with 1N hydrochloric acid, 50mL / time; wash the organic layer with anhydrous Na 2 SO 4 Dry, filter, concentrate and separate by column chromatography with 200-300 mesh silica gel, elute with a mixed solvent of petroleum ether: ethyl acetate: acetic acid (9.5: 0.5: 0.1) to obtain 7.9 g of Ia1 light yellow solid, 1 H-NMR (d 6 -DMSO): δppm 0.88-0.90 (6H, m), 1.15 (6H, s), 1.63 (4H, s), 1.82-1.91 (1H, m), 3.78-3.80 (2H, d, J=6.4Hz) , 3.95(2H, m), ...
Embodiment 2
[0159] Example 2 3,5-dihydroxy-4'-(4-methyl-4-carboxypentyloxy)-(E)-stilbene (Ia 2 )Synthesis
[0160] 1.13g Ia 1 Dissolved in 20mL of methanol, 4g of LiOH·H 2 O was made into 15 mL of aqueous solution. With stirring, the aqueous solution of LiOH was slowly added dropwise to Ia 1 In methanol solution, the reaction was stirred at room temperature for 4 days, and the reaction was tracked by TLC until the raw material point completely disappeared. Then add 80mL of water to the reaction solution, adjust the pH to 3-4 with 3M hydrochloric acid, extract 3 times with ethyl acetate (20mL / time); combine the extracts, wash with water until neutral, and wash the organic layer with anhydrous Na 2 SO 4 Dry, filter, and evaporate to dryness under reduced pressure to obtain Ia 2 0.78g of white solid, 1 H-NMR (d 6 -DMSO): δppm1.12 (6H, s), 1.61-1.63 (4H, m), 3.94-3.97 (2H, m), 6.12 (1H, m), 6.40 (2H, m), 6.89-7.00 (4H , m), 7.48-7.50 (2H, d, J=8.8Hz), 9.22 (2H, s), 12.15 (1H, s). ...
Embodiment 3
[0162] Example 3 3,5-dimethoxy-4'-(4-methyl-4-isobutoxycarbonylpentyloxy)-(E)-stilbene (Ia 3 )Synthesis
[0163] Will Ia 1 1.12g (2.72mmol) was dissolved in 20mL acetone, and 1.13g (8.19mmol) K was added successively 2 CO 3 and 0.35 mL (6.83 mmol) CH 3 1, Heating and reflux reaction under the protection of nitrogen for 24h, stop heating, after cooling, filter, wash the filter cake with acetone, combine the filtrate and lotion, after concentration, use 200-300 purpose silica gel to separate through column chromatography, use petroleum ether: ethyl acetate Esters (9:1) mixed solvent elution to give Ia 3 0.95g of colorless oil, 1 H-NMR (d 6 -DMSO): δppm0.85-0.90 (6H, m), 1.16 (6H, s), 1.63 (4H, m), 1.84-1.88 (1H, m), 3.79 (6H, m), 3.94 (2H, m ), 6.39-6.40 (1H, m), 6.74 (2H, br.s), 6.91 (2H, d, J = 8.8Hz), 7.02 (2H, d, J = 16Hz), 7.21 (1H, d, J = 16.4 Hz), 7.52 (2H, d, J = 8.4 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com