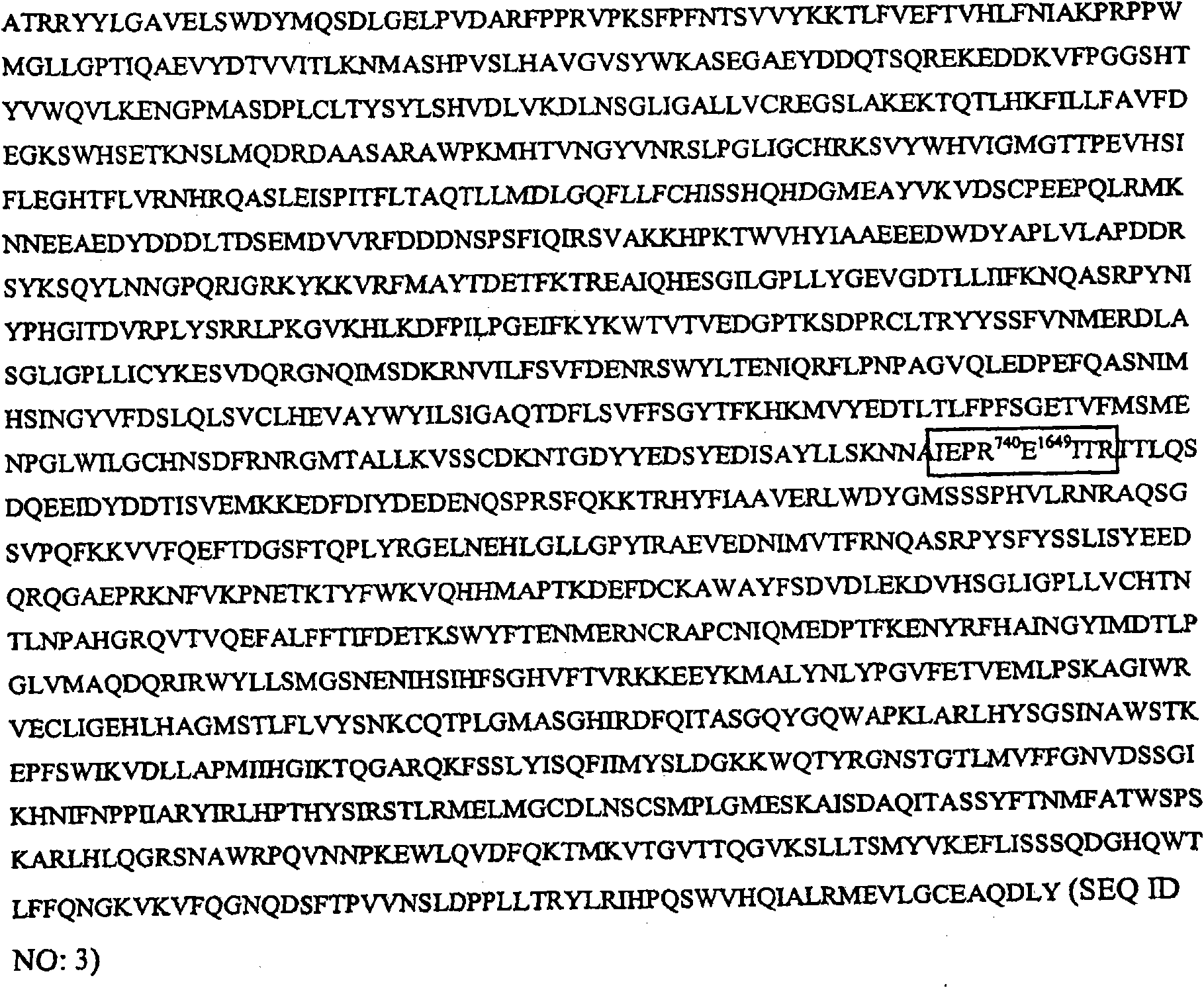Glycoconjugation of polypeptides using oligosaccharyltransferases
A polypeptide and glycosyl technology, applied in the field of polypeptide modification, can solve the problems of unsuitable attachment of modified groups and reduced biological activity of polypeptides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] I. abbreviation
[0033] PEG, poly(ethylene glycol); m-PEG, methoxy-poly(ethylene glycol); PPG, poly(propylene glycol); m-PPG, methoxy-poly(propylene glycol); Fuc, fucose or Fucosyl; Gal, galactose or galactosyl; GalNAc, N-acetylgalactosamine or N-acetylgalactosamine; Glc, glucose or glucosyl; GlcNAc, N-acetylglucosamine or N-acetyl Glucosamine; Man, mannose or mannosyl; ManAc, mannosamine acetate or mannosamine acetate; Sia, sialic acid or sialyl; and NeuAc, N-acetylceramide or N-acetylceramide base.
[0034] II. definition
[0035] Unless defined otherwise, all technical and scientific terms used herein generally have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Generally, the nomenclature and laboratory practices in cell culture, molecular genetics, organic and nucleic acid chemistry and hybridization used herein are those well known and commonly employed in the art. Standard techniques are used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com