Purification method of abiraterone acetate
A technology of abiraterone acetate and a purification method, which is applied in the field of purification of raw material drug abiraterone acetate, can solve the problems of unfavorable scale-up production, easy residual impurities, difficult filtration, etc., and achieve easy operation, simplified purification process, and reagent saving Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Preparation of Abiraterone Acetate Crude Product
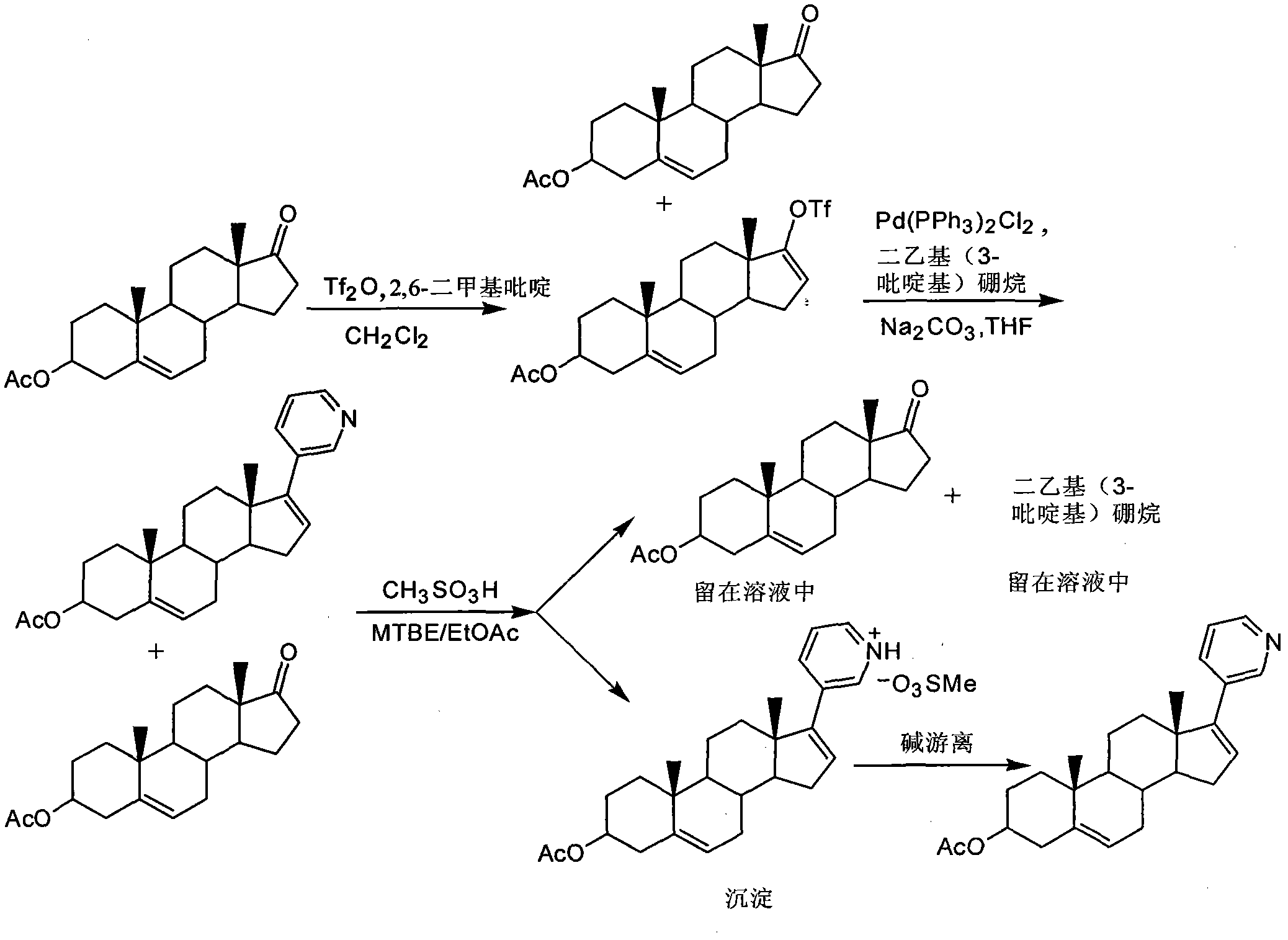
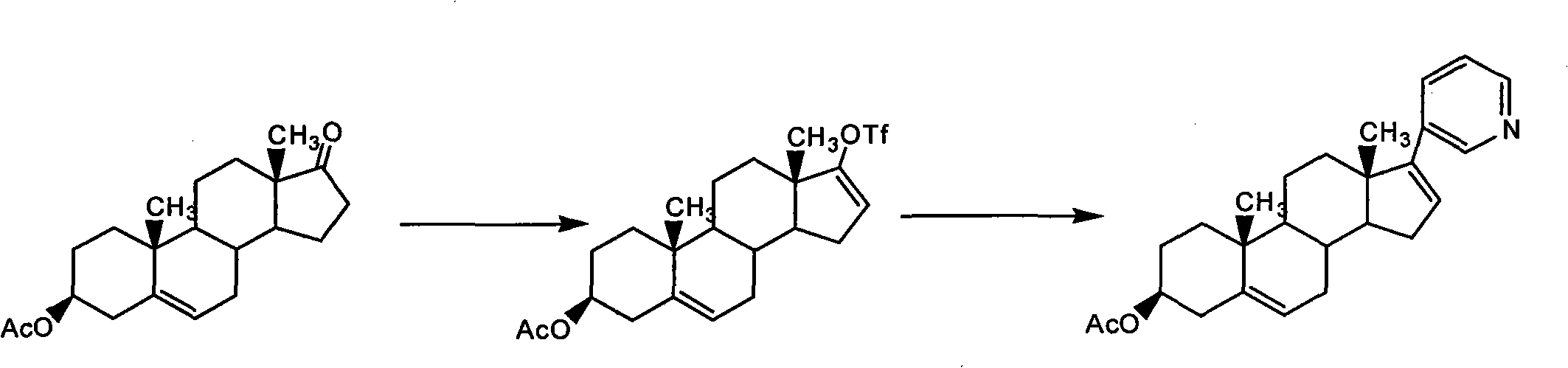
[0022] Add 100 g of dehydroepiandrosterone acetate to 1 L of dichloromethane, add 56 mL of trifluoromethanesulfonic acid under stirring, and stir the solution at room temperature for 5 minutes, then add 33.1 g of 2,6-lutidine within 25 minutes. 1L dichloromethane solution, and stirred at room temperature for 3.5 hours, after adding 1.5L water to quench the reaction, layered separation, the aqueous phase was extracted with 750mL dichloromethane, the organic phases were combined, and washed with 750mL 2N HCl solution, layered separation, Anhydrous Na 2 SO 4 After drying and treating the organic phase with 70 g of activated carbon for 10 minutes, suction filtration, the filtrate was concentrated under reduced pressure to obtain 127.5 g of brown oil;
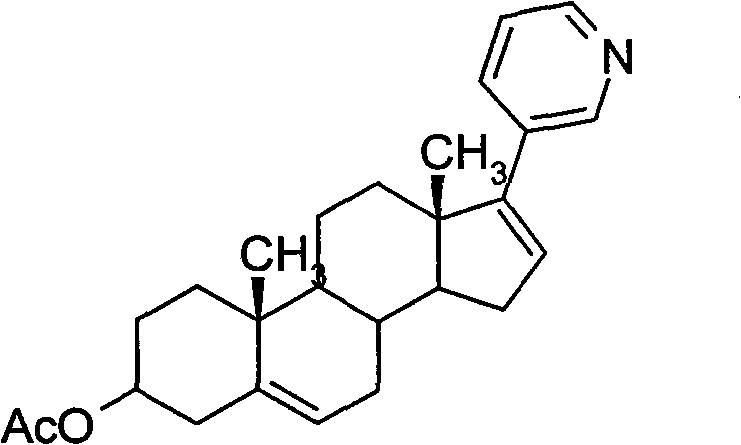
[0023] After dissolving 127.5g of brown oil in 1.3L of tetrahydrofuran (THF), 0.97g of Pd(PPh 3 ) 2 Cl 2 , 61.1 g diethyl (3-pyridyl) borane and 550 mL 2M N...
Embodiment 2
[0024] Embodiment 2 Purification of Abiraterone acetate crude product (trifluoromethanesulfonic acid is a salt-forming reagent)
[0025] Add 82.75g of abiraterone acetate crude oil to 250mL of ethyl acetate to completely dissolve it, then add it to a 1L three-necked flask, then add 250mL of methyl tert-butyl ether, stir and cool the mixture to -5~5°C, then add dropwise 21.35g of trifluoromethanesulfonic acid, after dripping, stirred at -5~5℃ for 1 hour, removed the ice bath, warmed up to room temperature, stirred at constant temperature for 2 hours, filtered with suction, and dried the filter cake to obtain 45.3g of off-white particles of albino acetate Biterone trifluoromethanesulfonate (HPLC purity 97.05%, only 1 impurity with content > 1%), yield 54.74%;
[0026] The above-mentioned abiraterone acetate trifluoromethanesulfonate was added to a 1L three-neck flask, and then 250mL of dichloromethane and 250mL of 20% Na 2 CO 3 Aqueous solution, after stirring for 20 minutes, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com