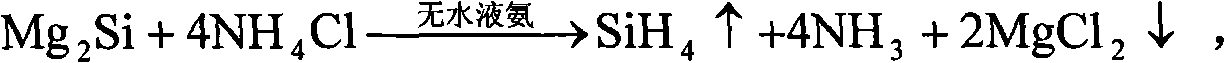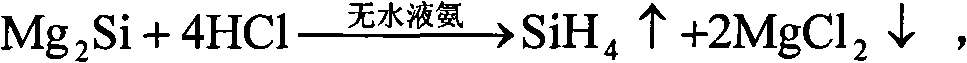Application of byproducts in preparation of silane through magnesium silicide process
A by-product, magnesium silicide technology, applied in the preparation/separation of ammonia, silicon hydride, chlorine/hydrogen chloride, etc., can solve the problems of large pollution, high energy consumption, and large recovery consumption, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
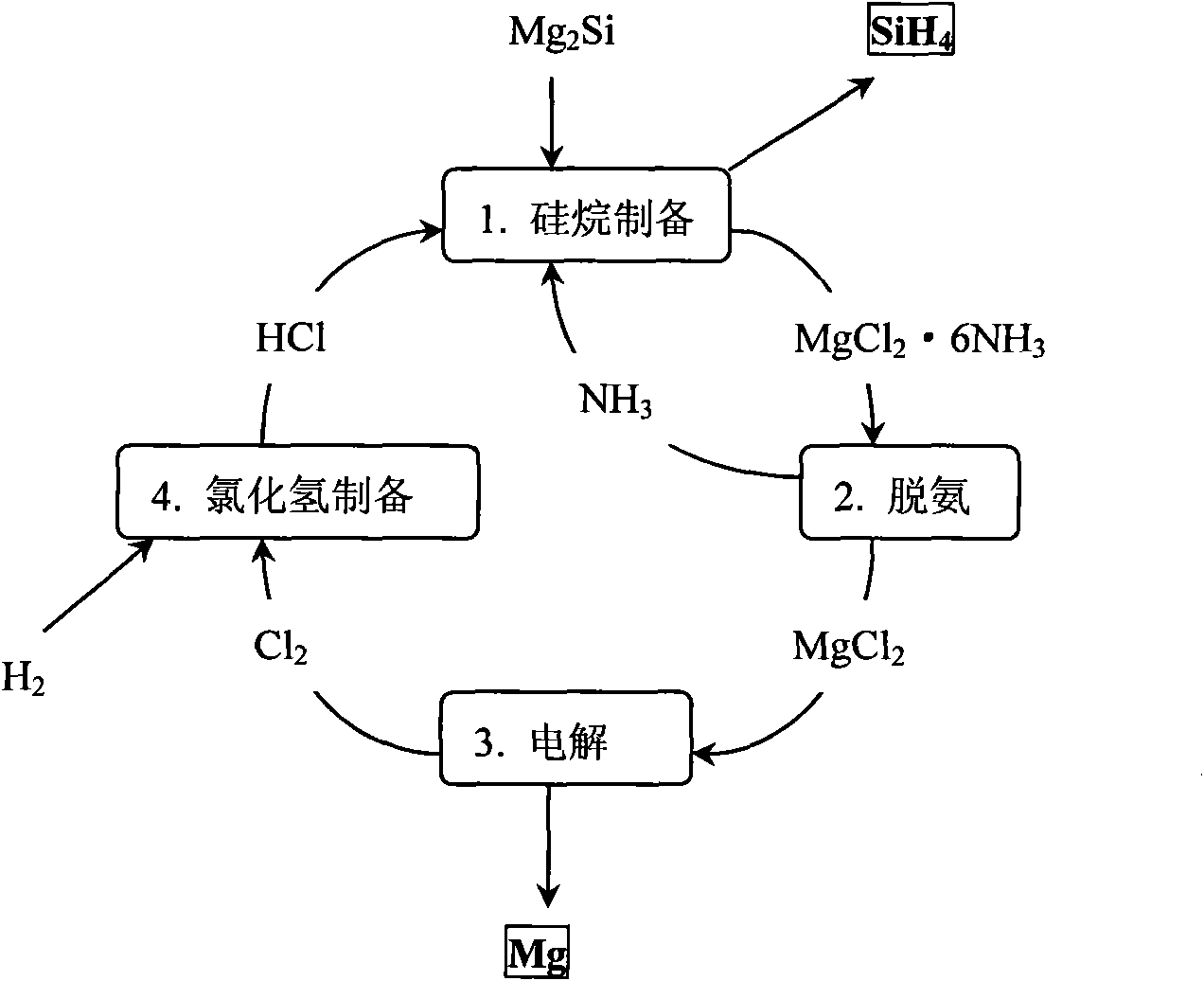
[0045] After weighing, 10mol Mg 2 Put Si into anhydrous liquid ammonia, keep the temperature of liquid ammonia at minus 30°C under one atmospheric pressure, and gradually and slowly introduce 40mol of anhydrous HCl, and after one hour of full reaction, gaseous SiH can be obtained 4 , and MgCl will be precipitated at the same time 2 6NH 3 By-products, the above reaction is carried out in an anhydrous closed environment. After taking out the by-products, after two hours of heating at 500°C, it decomposes to obtain anhydrous MgCl 2 and NH 3 gas, for MgCl 2 After weighing, it can be confirmed that it is 20mol of anhydrous MgCl 2 .
[0046] Anhydrous MgCl 2 Through molten electrolysis, the melting temperature is 950-1000°C, metal Mg can be obtained at the cathode, and 20mol of anhydrous Cl can be produced at the anode 2 .
[0047] The obtained 20mol of Cl 2 and 20mol of H 2 40 mol of anhydrous HCl that can be obtained through combustion reaction, after cooling to room te...
Embodiment 2
[0049] The metal magnesium powder obtained by electrolysis in Example 1 reacts with industrial silicon powder at high temperature to generate magnesium silicide Mg 2 Si, the reaction needs to be carried out in an atmosphere of hydrogen or argon, and the reaction temperature ranges from 600 to 1100°C. The amounts of metallic magnesium and industrial silicon are 20mol and 10mol respectively. There may be a small amount of loss or insufficient reaction in this reaction, and it may be necessary to increase the reactants as appropriate. get Mg 2 After Si, proceed according to the steps in Example 1. In this reaction, metal magnesium is recycled, and the product generated in the whole process is silane, and the raw materials that need to be supplemented are industrial silicon and hydrogen.
Embodiment 3
[0051] In the electrolytic magnesium industry, since the raw material MgCl is usually obtained 2 Both contain a large amount of crystal water, and dehydration must be carried out before the electrolysis of magnesium. One of the processes is to use ammonia for dehydration, which was first used by Nalco Chemical Company of the United States. The main idea is to use anhydrous ammonia, first by MgCl 2 ·6H 2 O to obtain MgCl 2 6NH 3 , and then deamination to obtain anhydrous and ammonia-free MgCl 2 , and then used in electrolysis, the specific process can be found on page 201 of "Magnesium Electrolysis Production Technology" edited by Zhang Yongjian. This process illustrates that MgCl 2 Ammonia removal is much easier than dehydration.
[0052] In obtaining anhydrous and ammonia-free MgCl 2 Afterwards, the steps described in Example 1 can be incorporated into the process cycle step. First electrolysis to obtain metal magnesium and Cl 2 , Cl 2 and H 2 Combustion to get HCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com