Method for preparing hindered phenol antioxygens by ester exchange process
A technology of hindered phenolic antioxidants and transesterification, which is applied in the field of preparing high-quality hindered phenolic antioxidants, which can solve the high requirements for the airtightness of reaction equipment, reduce the production efficiency of single reactors, and increase process operations Complexity and other issues, to achieve the effect of environmental protection, reduce impact, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
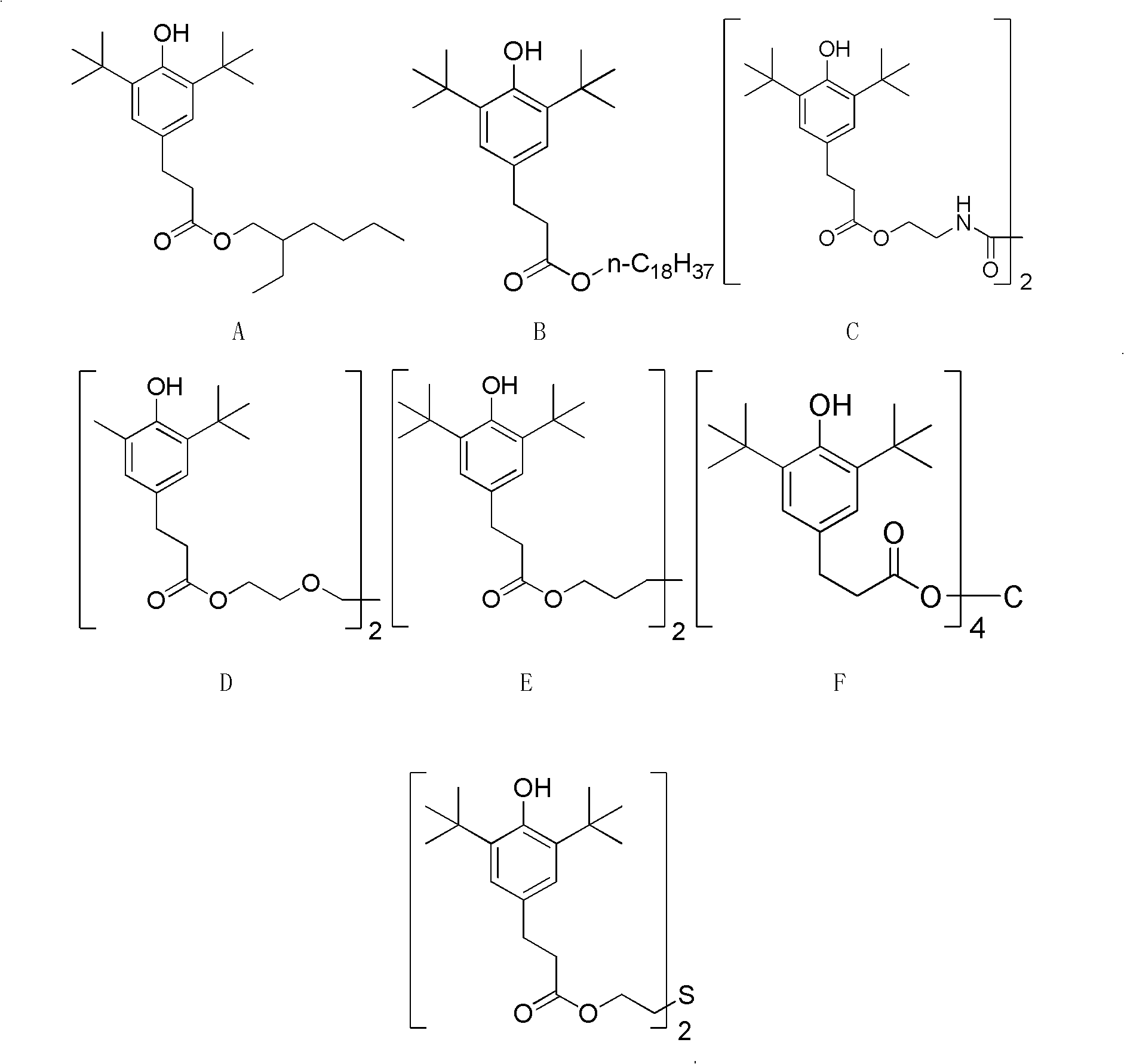
[0035] Preparation of 3-(3,5-di-tert-butyl-4-hydroxyphenyl) isooctyl propionate (chemical formula A)
[0036] In the 3000L reactor that rectifying tower is equipped with, add 3-(3,5-di-tert-butyl-4-hydroxyl phenyl) methyl propionate 1000.0kg (3.420kmol), isooctyl alcohol 448.2kg (3.442kmol ), toluene 600L, and pass into N 2 For protection, the reactor was heated to 130°C, light components were removed until the temperature at the top of the tower reached 110°C, and 3.0kg of monobutyltin triisooctoate was added. Reactor temperature is raised to 140 DEG C, adjust rectifying column top reflux ratio controller at the beginning, make in total reflux state; Treat tower top temperature to be stable at 63 DEG C, adjust reflux ratio (reflux flow rate: output) to be 35: 25 Methanol is produced in large quantities. When the tower top temperature reaches 75°C, adjust the rectification column reflux ratio controller reflux ratio (reflux: production volume) to 15:5 to reduce the productio...
Embodiment 2
[0039] Preparation of 3-(3,5-di-tert-butyl-4-hydroxyphenyl) n-octadecyl propionate (chemical formula B)
[0040] In the 3000L reactor that rectifying tower is equipped with, add 3-(3,5-di-tert-butyl-4-hydroxyl phenyl) methyl propionate 618.0kg (2.116kmol), n-stearyl alcohol 560.0kg (2.070 kmol), toluene 400L, and pass into N 2 for protection. Heat the reactor to 130°C, remove light components until the top temperature reaches 110°C, add 3.0 kg of aluminum isopropoxide, and heat the reactant to 140°C. At the beginning, adjust the reflux ratio controller at the top of the rectification column to make it in a full reflux state; when the temperature at the top of the column is stabilized at 63° C., adjust the reflux ratio (reflux rate: output) to 40:25 to produce a large amount of methanol. When the tower top temperature reaches 90°C, adjust the rectification column reflux ratio controller reflux ratio (reflux: production volume) to 25:5 to reduce the production volume so that t...
Embodiment 3
[0043] N, the preparation of N'-two (2-hydroxyethyl) oxamide two [3-(3,5-di-tert-butyl-4-hydroxyl phenyl) propionate] (chemical formula C)
[0044] In the 3000L reactor that rectifying tower is equipped with, add 3-(3,5-di-tert-butyl-4-hydroxyl phenyl) methyl propionate 1050.0kg (3.591kmol), N,N'-bis(2 -Hydroxyethyl) oxamide 300.0kg (1.702kmol), mixed xylene (boiling range 137 ~ 143 ℃) 600L, and pass into N 2 for protection. Heat the reactor to 150°C, remove light components until the top temperature reaches 137°C, add 7.0 kg of dibutyltin diacetate, continue to raise the temperature to 165°C, and adjust the rectification tower top reflux ratio controller at the beginning, so that It is in a state of total reflux; when the temperature at the top of the tower is stabilized at 65° C., adjust the reflux ratio (reflux rate: production volume) to 15:25 to produce a large amount of methanol. When the tower top temperature reaches 75°C, adjust the rectification column reflux ratio ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com