Productive technology of vanlillin by glyoxylic acid method
A production process, glyoxylic acid method technology, applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carbonyl compounds, etc., to achieve the effect of improving fractionation efficiency, reducing raw material consumption, and avoiding by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
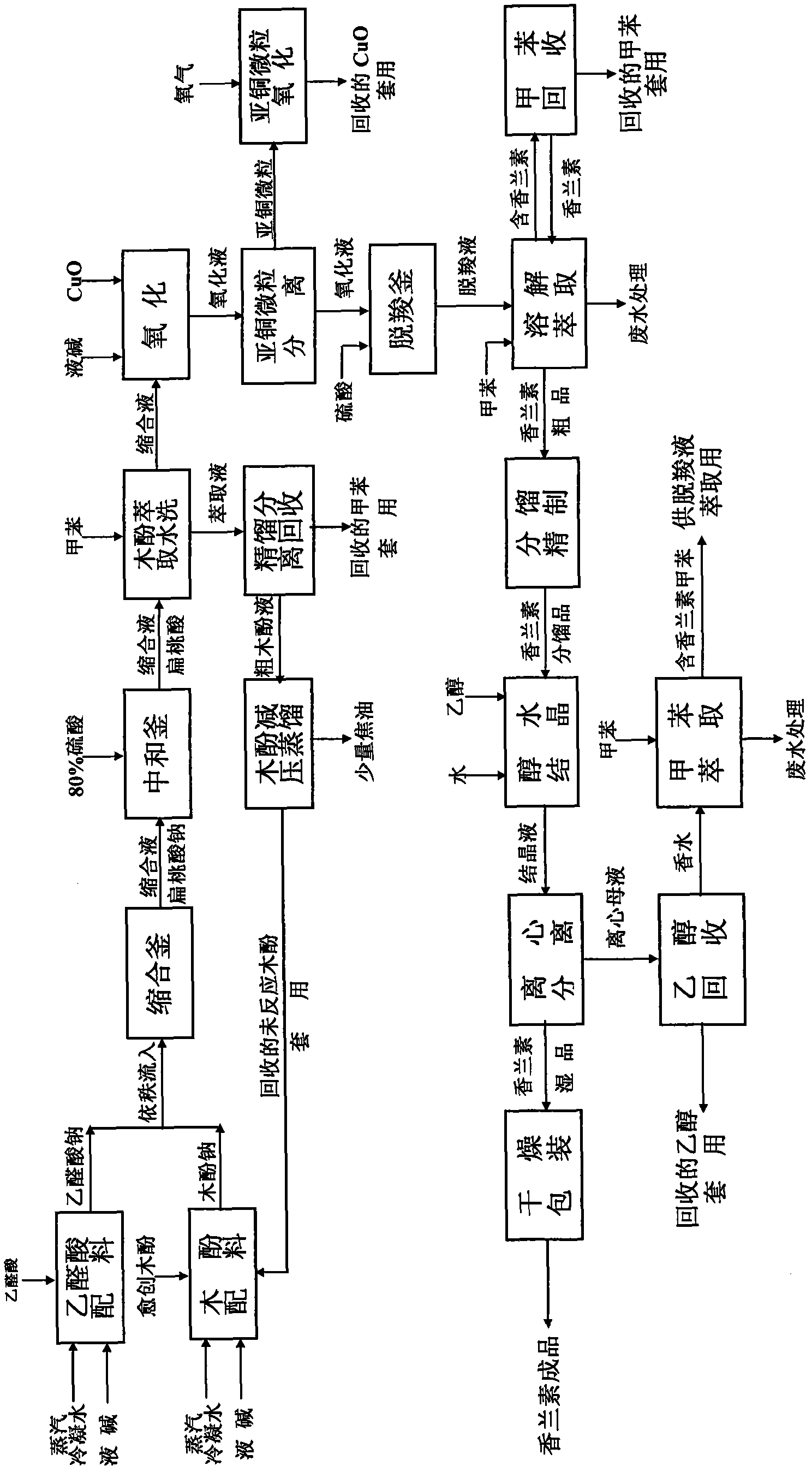
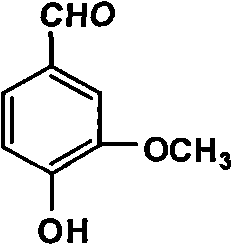
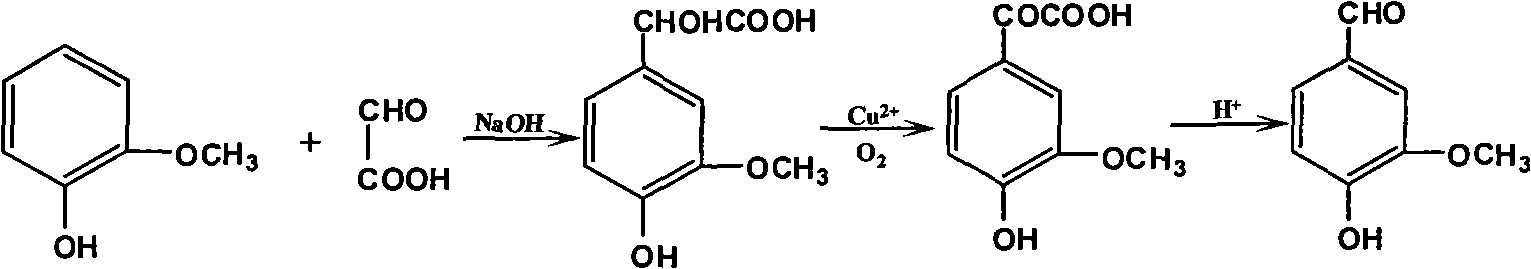
[0059] A glyoxylic acid production process of vanillin, which is characterized in that it includes the synthesis steps of condensation, oxidation and decarboxylation of guaiacol and glyoxylic acid; fractionation of crude vanillin and purification of alcohol water crystals process; in the synthesis process, it also involves the recycling process of recovering unreacted guaiacol, cuprous oxide and toluene.
[0060] The synthesis processes of condensation, oxidation and decarboxylation of the guaiacol and glyoxylic acid, etc.; also involve the recycling process of recovering unreacted guaiacol, cuprous oxide and toluene, including:
[0061] Add 30% liquid caustic soda to the batching kettle to convert guaiacol and glyoxylic acid into sodium salt respectively according to the feeding molar ratio of 1:0.77. Due to the exothermic reaction, the reaction temperature is required to be controlled at 14°C; then, the guaiacol Sodium lignophenate and sodium glyoxylate successively enter th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com