Method for synthesizing luteolin
A technology of luteolin and ethanol, which is applied in the synthesis field of preparing luteolin, can solve the problems of low extraction rate, and achieve the effects of high synthesis efficiency, high yield, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
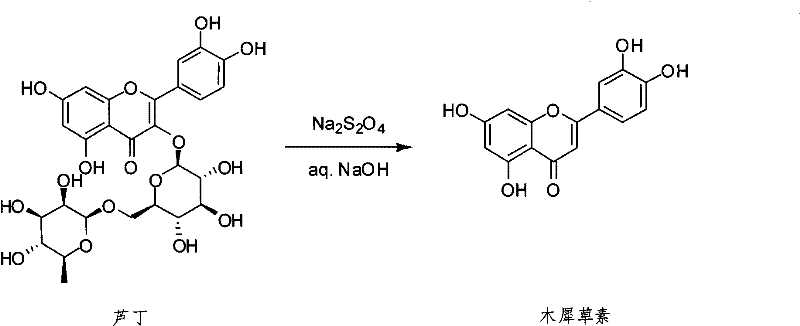
[0016] synthetic route:
[0017]
[0018] Preparation: Add 600 grams of water to a 1L three-necked bottle, then add 90 grams of sodium hydroxide, stir to dissolve it, wait until the temperature of the reaction bottle drops to 20-30 degrees, slowly add 30 grams of rutin, and continue stirring for 30 Minutes, until the rutin is completely dissolved, then heat up to 50 degrees, add 65 grams of sodium hydrosulfite, stir at this temperature for 45 minutes, heat up to 100 degrees, and stir for 12 hours. HPLC detects that the reaction is over, cool the reaction bottle, drop dilute hydrochloric acid or dilute sulfuric acid between 20-25 degrees, adjust the pH to 3-4, then let it stand for 10 hours, filter, wash with water, and dry to obtain 11.5 grams of crude Osmanthus osmanthus Grass.
[0019] Refining: Add 11.5 grams of crude luteolin to 200 grams of ethanol, heat to dissolve, then concentrate to get 120 grams of ethanol, place the remaining solution at 0-10 degrees for 8 hours...
Embodiment 2
[0021] Preparation: Clean the 500L glass-lined reaction kettle, add 300kg of water to the kettle, then add 45kg of caustic soda, turn on the cooling water to lower the temperature to below 25 degrees, add 15kg of rutin in 3 batches, and then stir for 1 Hours to dissolve all the rutin. Then heat up to 45-50 degrees, slowly add 32.5 kg of hydrochloric acid, stir at this temperature for 30 minutes, then heat up to reflux for 12 hours, cool the reactor to below 20 degrees, drop dilute hydrochloric acid until the pH reaches 3-4, and then Let it stand for 10 hours, pass through plate and frame filter press, wash the product obtained once with water, and dry to obtain 6 kg of luteolin.
[0022] Refining: dissolving 6 kg of luteolin in 100 kg of ethanol, then steaming out 55 kg of ethanol, cooling the remaining solution to 0-5 degrees, standing for 10 hours for crystallization, and obtaining 4 kg of luteolin with a purity of 95.6% , the yield was 56.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com