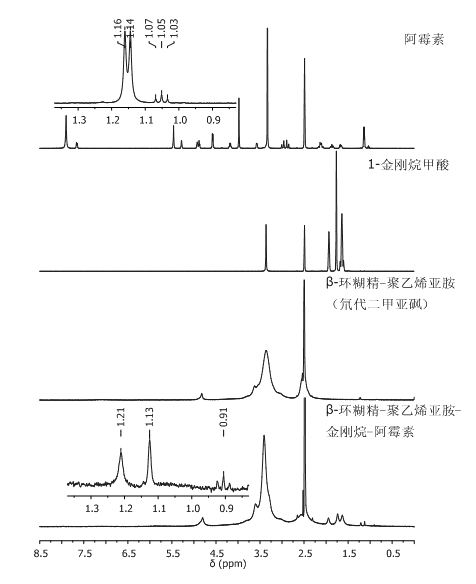
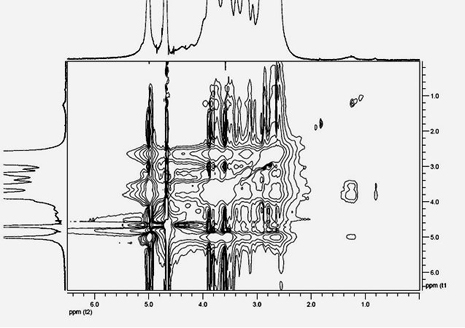
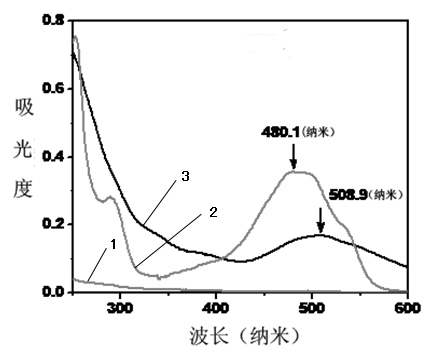
Cyclodextrin-polyethyleneimine-mediated supramolecular delivery system and preparation method
A polyethylenimine and cyclodextrin technology, which is applied in the field of non-viral gene transfection vectors, can solve the problems of low targeting, high cytotoxicity, limited application scope of viral vectors, etc., and achieves low toxicity, increased functions, Strong transformative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Take β-cyclodextrin as an example.
[0061] 1) Preparation of polyethyleneimine-cyclodextrin polymer:
[0062]Weigh 5 grams of β-cyclodextrin and dissolve it in 50 milliliters of dimethyl sulfoxide, weigh 10 grams of N,N'-carbonyldiimidazole and dissolve it in 20 milliliters of dimethyl sulfoxide, and store in a dark place under the protection of nitrogen Mix the N,N'-carbonyldiimidazole solution and the cyclodextrin solution, add 300 microliters of triethylamine, stir and react for 2 hours, weigh 15 grams of polyethyleneimine (PEI600) with a molecular weight of 600, and dissolve it in 30 milliliters In dimethyl sulfoxide, the solution is slowly added dropwise to the activated cyclodextrin solution in a dark place under the protection of nitrogen, and the dropwise addition is completed within 2 hours. After the dropwise addition is completed, the reaction is continued for 3 hours. After the reaction, the solution was dialyzed in a 8000-14000 dialysis bag for...
Embodiment 2
[0081] Embodiment 2: Taking α-cyclodextrin as an example
[0082] 1) Preparation of polyethyleneimine-cyclodextrin polymer:
[0083] Weigh 10 grams of α-cyclodextrin and dissolve it in 60 milliliters of dimethyl sulfoxide, weigh 20 grams of N,N'-carbonyldiimidazole and dissolve it in 30 milliliters of dimethyl sulfoxide, and store in a dark place under the protection of nitrogen Mix the N,N'-carbonyldiimidazole solution and the cyclodextrin solution, add 300 microliters of triethylamine, stir and react for 2 hours, weigh 10 grams of polyethyleneimine (PEI1200) with a molecular weight of 1200, and dissolve it in 20 ml In dimethyl sulfoxide, the solution is slowly added dropwise to the activated cyclodextrin solution in a dark place under the protection of nitrogen, and the dropwise addition is completed within 2 hours. After the dropwise addition is completed, the reaction is continued for 3 hours. After the reaction, the solution was dialyzed in a 8000-14000 dialysis bag for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com