Method for preparing 1,3-butadiene from normal butene by using continuous-flow dual-bed reactor
A flow reactor, butadiene technology, applied in chemical instruments and methods, dehydrogenation to hydrocarbons, carbon compound catalysts, etc., can solve the problems of complex catalyst structure, side reactions, large energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
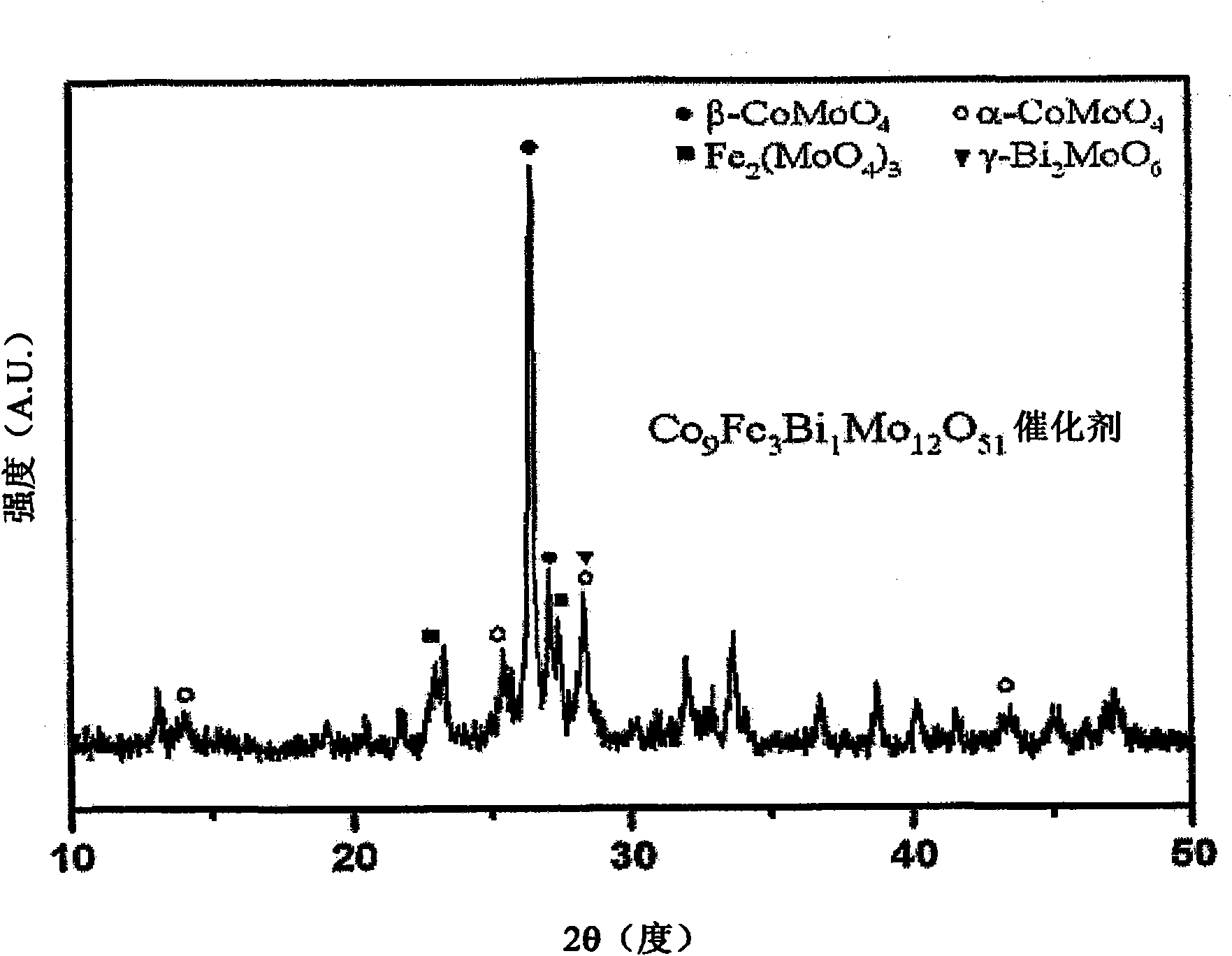
[0056] Multicomponent bismuth molybdate (Co 9 Fe 3 Bi 1 Mo 12 O 51 ) Preparation of catalyst
[0057] Cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O) as cobalt precursor, iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) as iron precursor, bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) as bismuth precursor, ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) as a molybdenum precursor. All precursors are readily soluble in distilled water except bismuth nitrate pentahydrate, which is readily soluble in strongly acidic solutions. Therefore, bismuth nitrate pentahydrate was dissolved alone in a solution formed by adding nitric acid to distilled water.
[0058] In order to prepare the multi-component bismuth molybdate catalyst, the molar ratio of cobalt:iron:bismuth:molybdenum was set to 9:3:1:12. 7.94 grams of cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O) and 3.66 g of iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) was di...
preparation example 2
[0064] Multicomponent bismuth molybdate catalysts containing manganese or nickel as metal components with divalent cations Chemical preparation
[0065] For the preparation of multicomponent bismuth molybdate catalysts containing manganese or nickel as the metal component with divalent cations, 7.83 g of manganese nitrate hexahydrate (Mn(NO 3 ) 2 ·6H 2 O) and 7.93 grams of nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O). The preparation conditions of the multi-component bismuth molybdate catalyst are the same as those in Preparation Example 1, except that the types and contents of the precursors with divalent cations are different. The prepared multi-component bismuth molybdate catalyst was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES). From the ICP-AES analysis results of the multi-component bismuth molybdate catalyst, it can be seen that the required amount of metal precursors was accurately co-precipitated, and the error was within...
preparation example 3
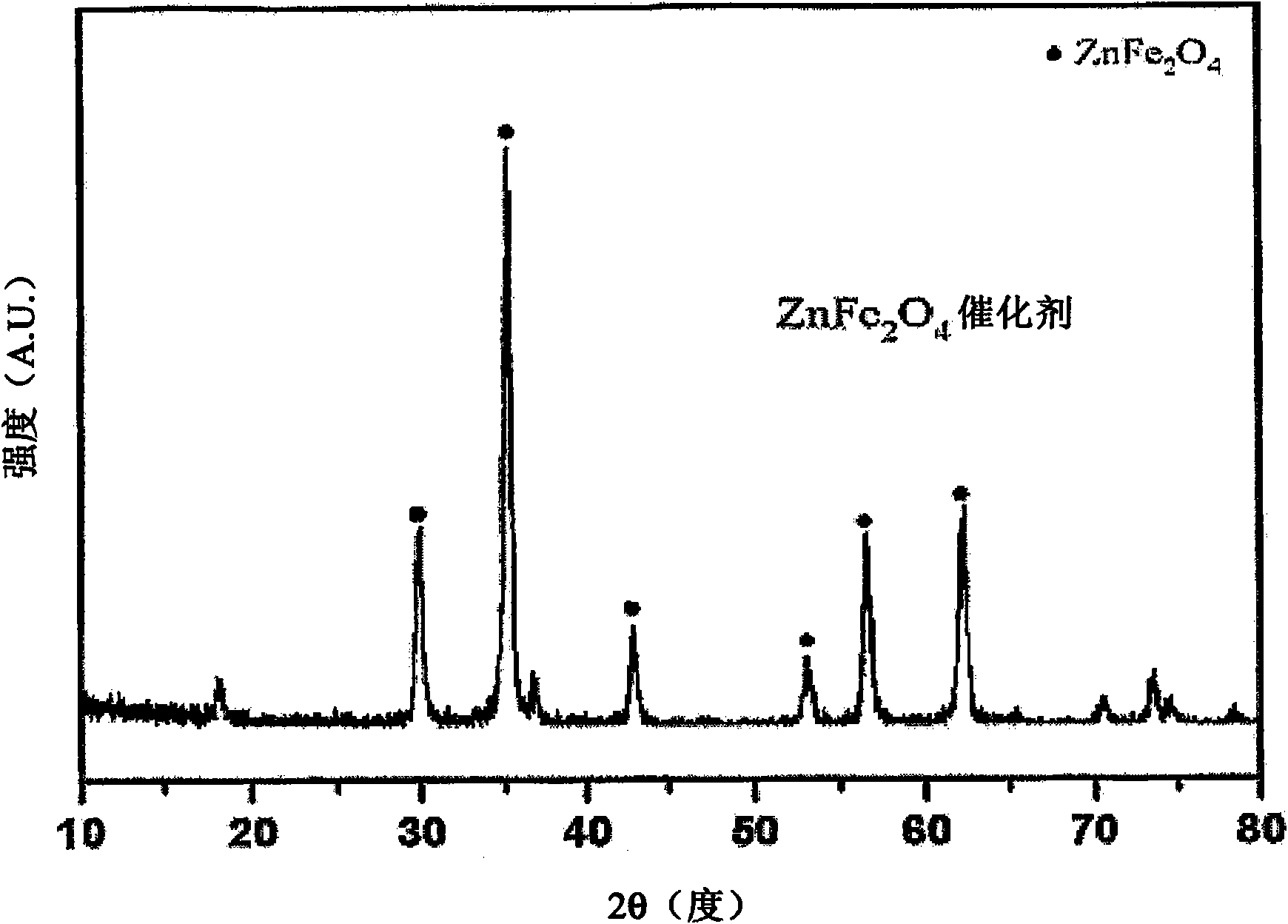
[0077] Zinc ferrite (ZnFe 2 O 4 ) Preparation of catalyst
[0078] Zinc chloride (ZnCl 2 ) as the zinc precursor, ferric chloride hexahydrate (FeCl 3 ·6H 2 O) as iron precursors. To prepare the zinc ferrite catalyst, 1.42 grams of zinc chloride and 5.61 grams of ferric chloride hexahydrate were dissolved in distilled water (100 ml) and stirred to form an aqueous precursor solution. After the precursor was completely dissolved, the aqueous solution of the precursor was added dropwise to distilled water (100ml), and at the same time, a 3M aqueous sodium hydroxide solution was added therein so that the pH of the co-precipitation solution was 9, thereby forming a mixed solution.
[0079] The mixed solution was sufficiently stirred with a magnetic stirrer at room temperature for 12 hours, and then left to stand at room temperature for 12 hours to perform phase separation, thereby precipitating the mixed solution. The precipitated mixed solution was filtered using a v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com