Immobilized cyclodextrin glucoside transferase and preparation method and application thereof
A technology of glucoside and cyclodextrin, applied in the direction of immobilization on/in organic carrier, chemical industry, sustainable manufacturing/processing, etc., can solve the problem of poor thermal stability and storage stability, large loss of enzyme activity, immobilization To solve the problem of high chemical cost, achieve the effects of high thermal stability and storage stability, cost reduction, and easy extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
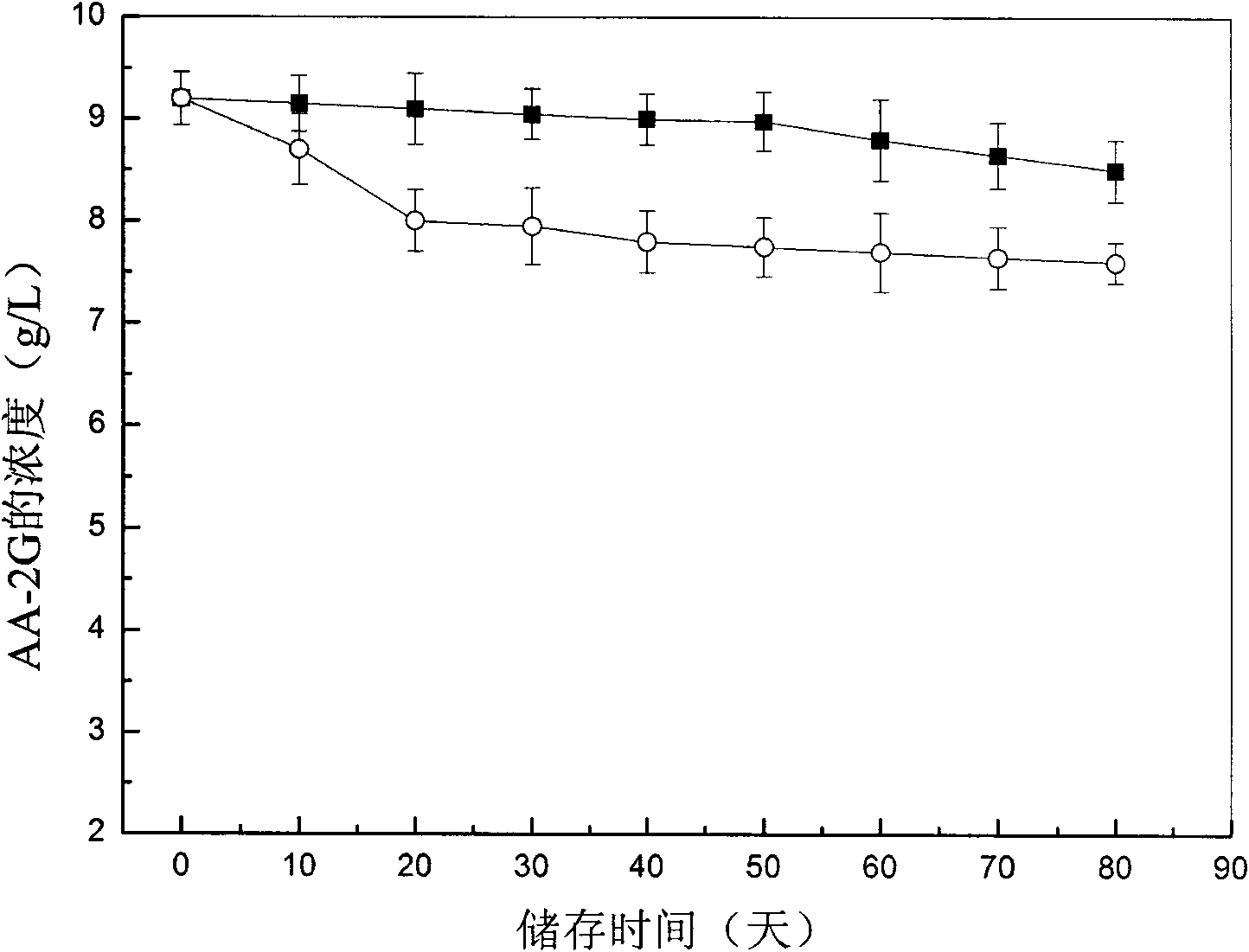
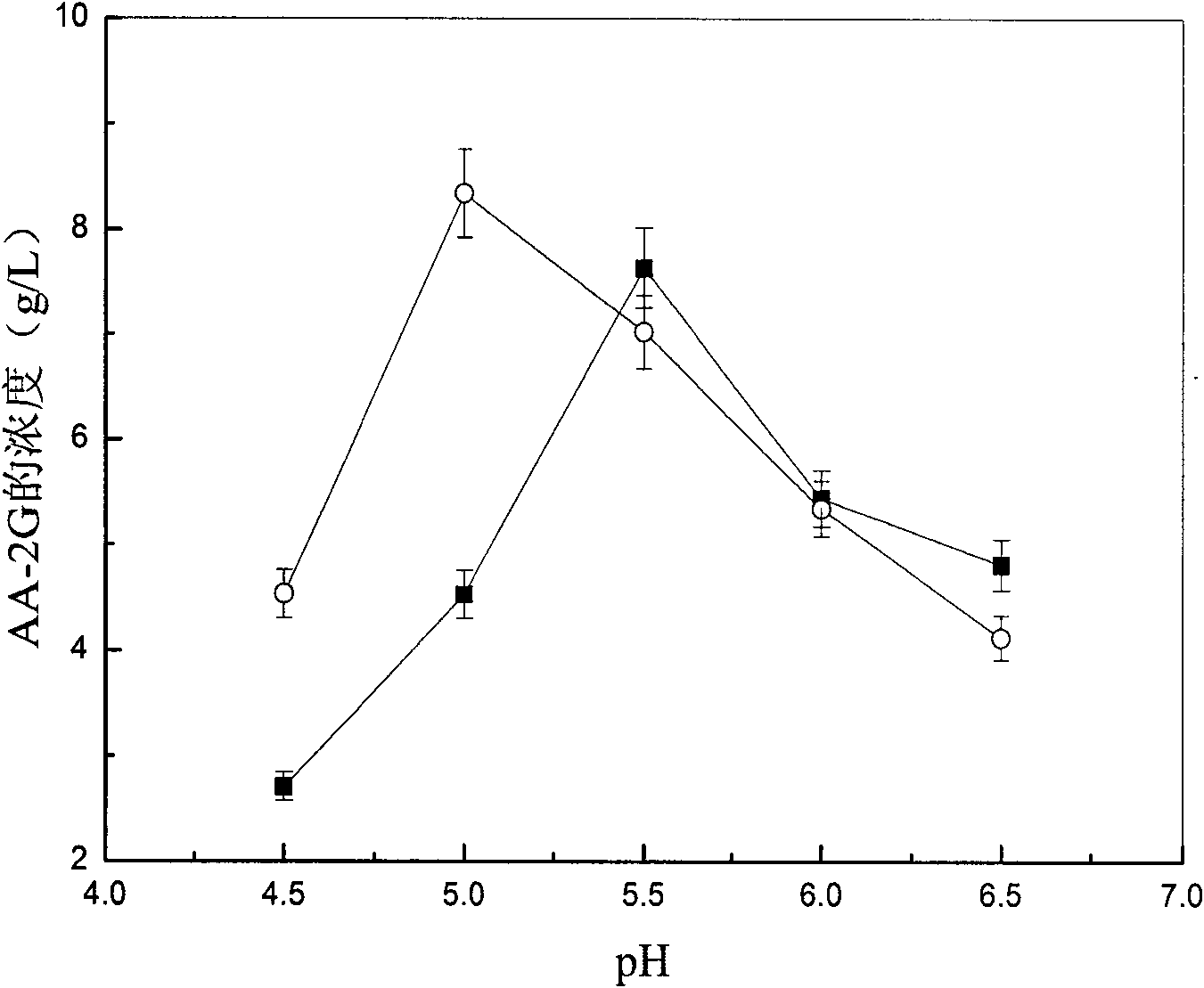
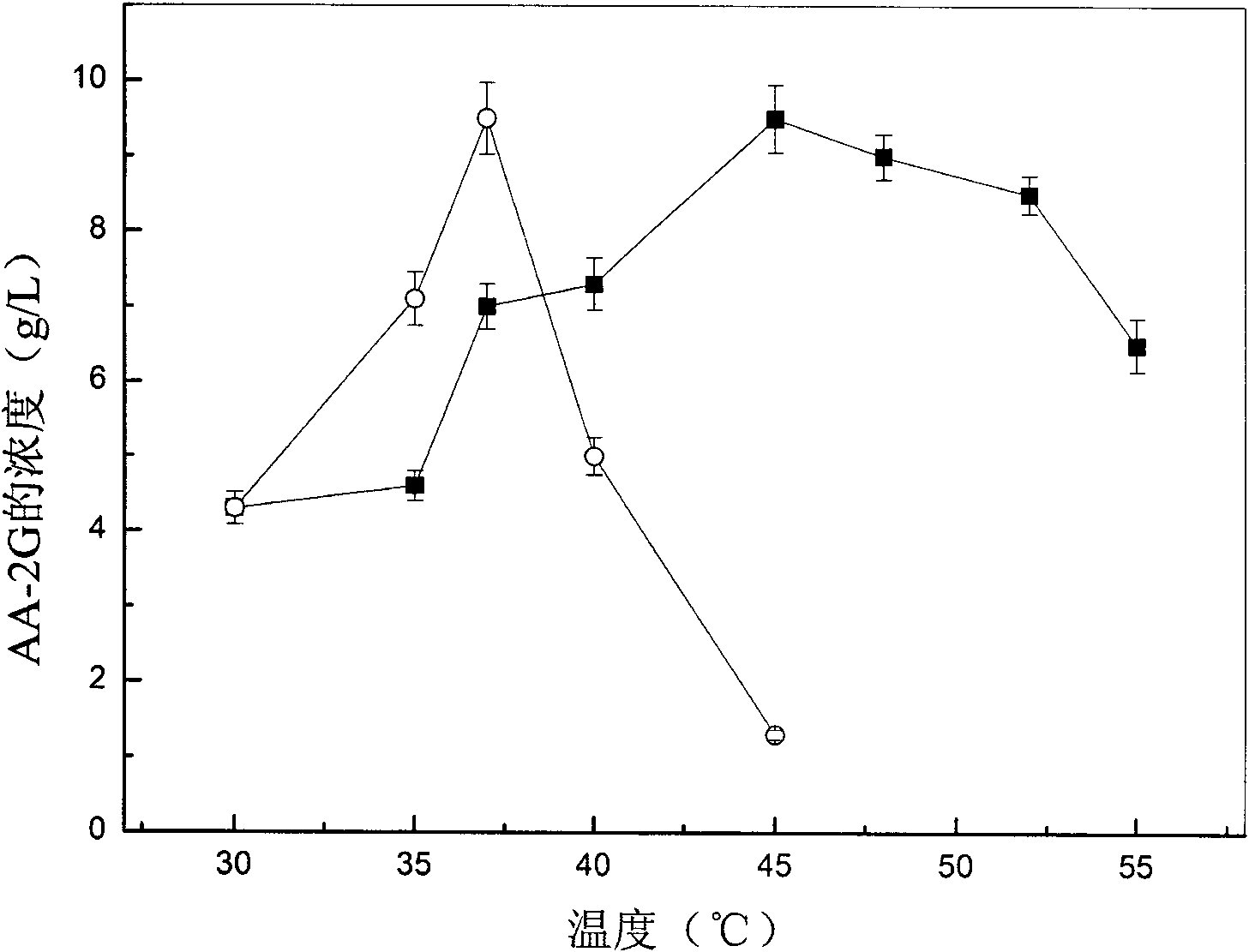
[0031] Embodiment 1: 2-O-glucosyl ascorbic acid assay method (HPLC method)
[0032] After the transformation liquid was centrifuged at 8000 rpm for 5 min, 1 mL of the supernatant was taken, and the volume was made up to a 10 mL volumetric flask with distilled water. Then, the samples were injected after filtration with a 0.22 μm filter membrane. Substitute the peak area into the standard curve to calculate the concentration of AA-2G.
[0033] Liquid phase instrument: Agilent 1200 liquid phase instrument
[0034] Column: Agilent SB-Aq
[0035] Flow rate: 0.5mL / min
[0036] Detector: DAD
[0037] Column temperature: 25°C
[0038] Detection wavelength: 238nm
[0039] Mobile phase: 6.86g KH 2 PO 4 , 5mL of methanol, dissolved in 1000mL of ultrapure water, and adjusted to pH 2.0 with phosphoric acid.
[0040] Definition of enzyme activity: the enzyme activity is expressed by the content of glucosyl ascorbic acid (g / L) in the transformation solution, and AA-2G is used as th...
Embodiment 2
[0041] Embodiment 2: Immobilization method 1 of cyclodextrin glucosidyl transferase
[0042] Put 0.02g of molecular sieve into 10mL of enzyme solution, then put it into a shaker at 16°C, shake at 150rpm for 10h to fully absorb, then add 100μL of 25% glutaraldehyde solution to mix and crosslink for 4h, then add 0.2g of sodium alginate and mix evenly. Put it in a refrigerator at 4°C overnight for degassing, and then use a syringe to drop it into 3% CaCl2 for hardening for 4 hours. The collected capsules were rinsed with distilled water until no protein was precipitated, and the rinsed capsules were stored in glycerin in a refrigerator at 4°C for later use.
Embodiment 3
[0043] Embodiment 3: Immobilization method 2 of cyclodextrin glucosidyl transferase
[0044] Add 0.06g molecular sieves to 10mL enzyme solution, then put it into a shaker at 16°C, shake at 150rpm for 8h for full adsorption, then add 100μL of 25% glutaraldehyde solution to mix and crosslink for 4h, then add 0.2g sodium alginate and mix evenly. Put it in a refrigerator at 4°C overnight for degassing, and then use a syringe to drop it into 6% CaCl2 for hardening for 2 hours. The collected capsules were rinsed with distilled water until no protein was precipitated, and the rinsed capsules were stored in glycerin in a refrigerator at 4°C for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com