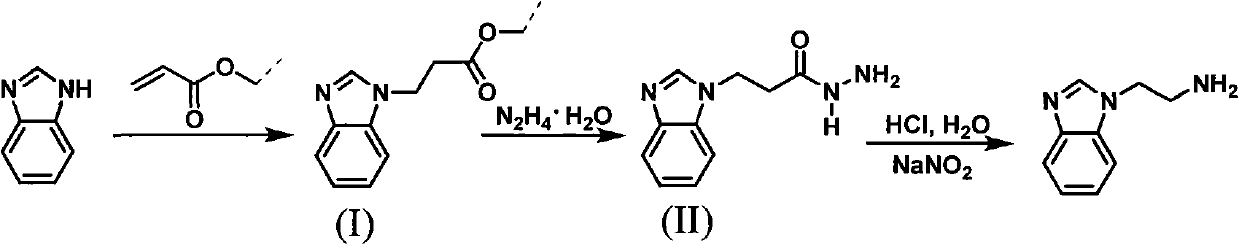
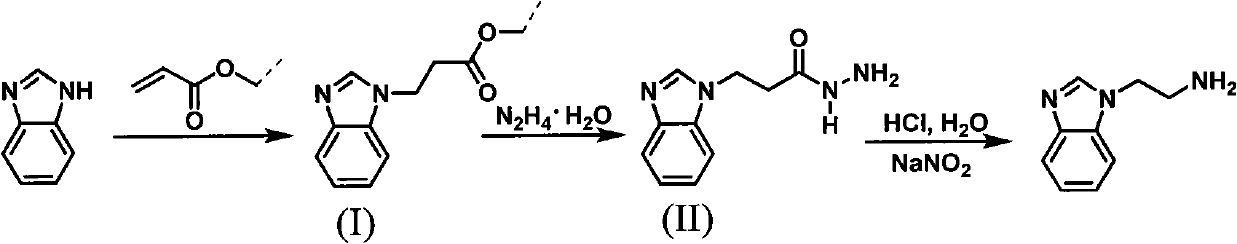
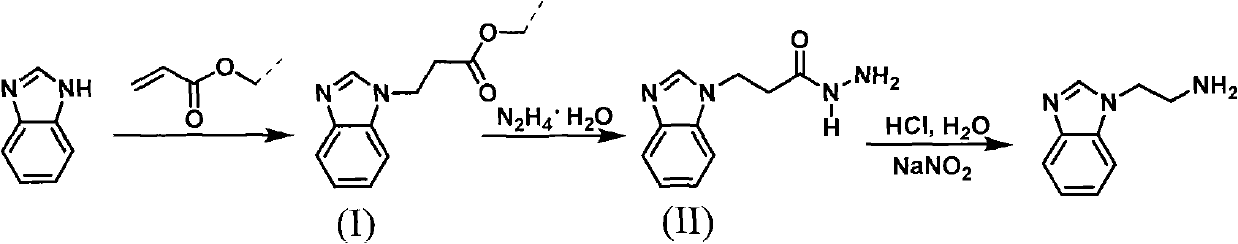
Method for synthesizing 2-(1-benzimidazolyl) ethylamine
A benzimidazole-based, synthetic method technology, applied in the field of practical synthesis of 2-ethylamine, can solve the problems of post-processing difficulties, low total yield, inconvenient reaction operation, etc., and achieve reasonable reaction process design and high total yield High, smooth response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] (1), the synthesis of intermediate (I) 2-(1-benzimidazolyl) methyl propionate
[0015]
[0016] Add benzimidazole (5.9g, 50mmol), triethylamine (0.15g, 1.5mmol), methanol (50ml) successively into a 100mL two-necked flask equipped with a reflux condenser and a stirrer, stir to dissolve, and heat up to 40°C. Methyl acrylate (5.2 g, 60 mmol) was added dropwise. The end point of the reaction was monitored by TLC ((developer chloroform:methanol=9:1 / v:v). After the reaction was completed, excess methyl acrylate was distilled off to obtain a white paste solid compound (9.9 g), with a yield of 97%. 1 H NMR (400Hz, CDCl 3 ): δ2.88(t, J=6.4Hz, 2H), 3.67(s, 3H), 4.52(t, J=6.4Hz, 2H), 7.27-7.34(m, 2H), 7.39-7.41(m, 1H), 7.80-7.83(m, 1H), 7.99(s, 1H).
[0017] (2), the synthesis of intermediate (II) 2-(1-benzimidazolyl) propionyl hydrazide
[0018]
[0019] Add 85% hydrazine hydrate (5mL, 100mmol) and methanol (20ml) to a 100mL two-necked flask equipped with a reflux conde...
Embodiment 2
[0024] (1), the synthesis of intermediate (I) 2-(1-benzoimidazolyl) ethyl propionate
[0025]
[0026] Add benzimidazole (5.9g, 50mmol), tetrabutylammonium hydroxide (0.26g, 1.0mmol), ethanol (50ml) successively into a 100mL two-necked flask equipped with a reflux condenser and a stirrer, stir to dissolve, and heat up to 60°C , ethyl acrylate (8.3 g, 75 mmol) was added dropwise. The end point of the reaction was monitored by TLC ((developer chloroform:methanol=9:1 / v:v). After the reaction was completed, excess methyl acrylate was distilled off to obtain a white paste solid compound (10.4 g), with a yield of 95%.
[0027] (2), the synthesis of intermediate (II) 2-(1-benzimidazolyl) propionyl hydrazide
[0028]
[0029] Add 85% hydrazine hydrate (4mL, 80mmol) and ethanol (20ml) into a 100mL two-necked flask equipped with a reflux condenser and a stirrer, and add the intermediate (I) 2-(1-benzimidazolyl) dropwise under stirring. ) Ethyl propionate (8.2g, 40mmol) in ethano...
Embodiment 3
[0033] (1), the synthesis of intermediate (I) 2-(1-benzimidazolyl) methyl propionate
[0034]
[0035] Add benzimidazole (1.2g, 10mmol), triethylamine (0.05g, 0.5mmol) and ethanol (20ml) successively into a 100mL two-necked flask equipped with a reflux condenser and a stirrer, stir to dissolve, and heat up to 40°C. Methyl acrylate (1.1 g, 12 mmol) was added dropwise. The end point of the reaction was monitored by TLC ((developer chloroform:methanol=9:1 / v:v). After the reaction was completed, excess methyl acrylate was distilled off to obtain a white paste solid compound (2.0 g), with a yield of 98%.
[0036] (2), the synthesis of intermediate (II) 2-(1-benzimidazolyl) propionyl hydrazide
[0037]
[0038] Add 85% hydrazine hydrate (1mL, 20mmol) and n-propanol (20ml) in a 100mL two-necked flask equipped with a reflux condenser and a stirrer, and add the mixture containing intermediate (I) 2-(1-benzimidazole) dropwise under stirring. Base) ethyl propionate (2.1 g, 10 mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com