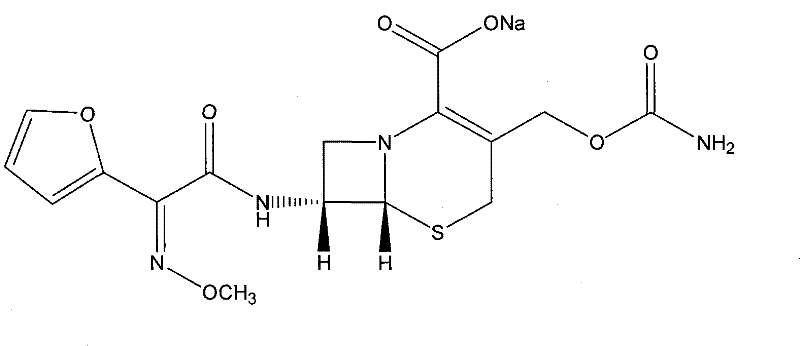
Method for recrystallizing cefuroxime sodium
A cefuroxime sodium and recrystallization technology, which is applied in the field of medicine, can solve the problems of being unsuitable for the production of sterile raw materials, deepening the color of cefuroxime sodium, and cumbersome operations, etc., achieving low cost, high recrystallization efficiency, and increased yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a. Preparation of crude solution: In a 500mL round bottom flask, add 200mL of water for injection at 15°C, 35.0g of crude cefuroxime sodium raw material (color grade No. 6), stir until all the solids are dissolved, add 1.1g of activated carbon, and control the temperature of the solution at 10-15°C, stir for 30 minutes, filter, wash the filter cake with 10mL water for injection at 15°C, combine the filtrates, add 10% acetic acid aqueous solution dropwise thereto to adjust the pH value to 6.5, and set aside;
[0023] b. Preparation of crystallization solvent: prepare 1015 mL of mixed liquid with methanol and acetone at a volume ratio of 0.5:10, stir evenly, and set aside;
[0024] c. Crystallization: Add the crude product solution obtained in step a dropwise to the crystallization solvent obtained in step b, and stir while adding, and a white solid is precipitated. After the dropwise addition, continue to add 560 mL of acetone dropwise. The temperature of the solution is ...
Embodiment 2
[0027] a. Preparation of crude solution: In a 500mL round bottom flask, add 200mL of water for injection at 15°C, 35.0g of crude cefuroxime sodium raw material (color grade No. 6), stir until all the solids are dissolved, add 1.1g of activated carbon, and control the temperature of the solution 10-15°C, stir for 30 minutes, filter, wash the filter cake with 10mL water for injection at 15°C, combine the filtrates, add 10% acetic acid aqueous solution dropwise thereto to adjust the pH value to 6.7, and set aside;
[0028] b. Preparation of crystallization solvent: prepare 1015 mL of mixed liquid with methanol and acetone in a volume ratio of 1:10, stir evenly, and set aside;
[0029] c. Crystallization: Add the crude product solution obtained in step a dropwise to the crystallization solvent obtained in step b, and stir while adding, and a white solid is precipitated. After the dropwise addition, continue to add 560 mL of acetone dropwise. The temperature of the solution is contr...
Embodiment 3
[0032] a. Preparation of crude solution: In a 500mL round bottom flask, add 200mL of water for injection at 10°C, 35.0g of crude cefuroxime sodium raw material (color grade No. 7), stir until all the solids are dissolved, add 1.1g of activated carbon, and control the temperature of the solution 10-15°C, stir for 30 minutes, filter, wash the filter cake with 10mL water for injection at 10°C, combine the filtrates, add 20% acetic acid aqueous solution dropwise to adjust the pH value to 7.0, and set aside;
[0033] b. Preparation of crystallization solvent: prepare 1015mL of mixed liquid with methanol and acetone at a volume ratio of 1.5:10, stir evenly, and set aside;
[0034] c. Crystallization: Add the crude product solution obtained in step a dropwise to the crystallization solvent obtained in step b, stir while adding, and a white solid is precipitated. After the dropwise addition, continue to add 560 mL of acetone dropwise. The temperature of the solution is controlled durin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com