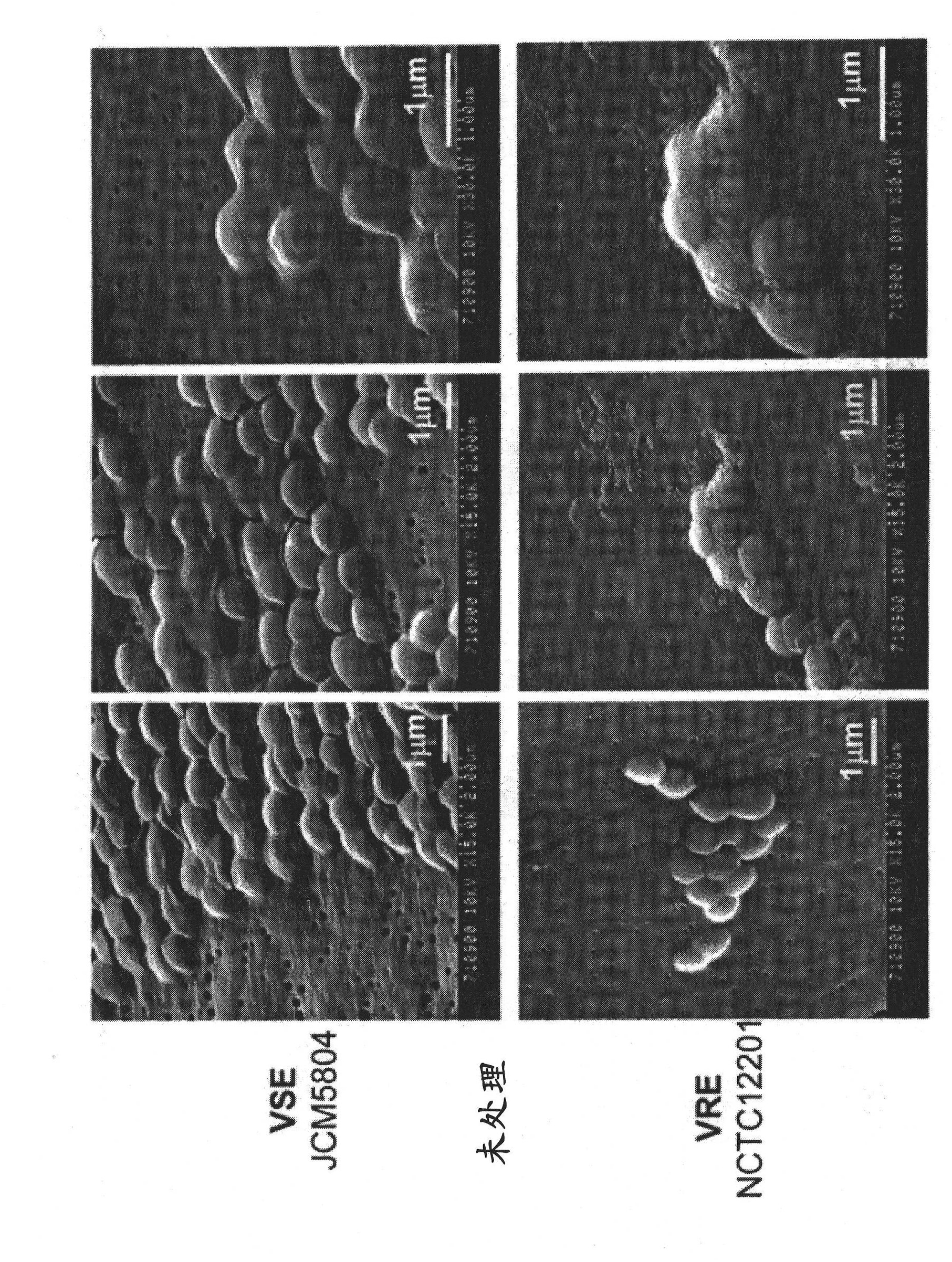
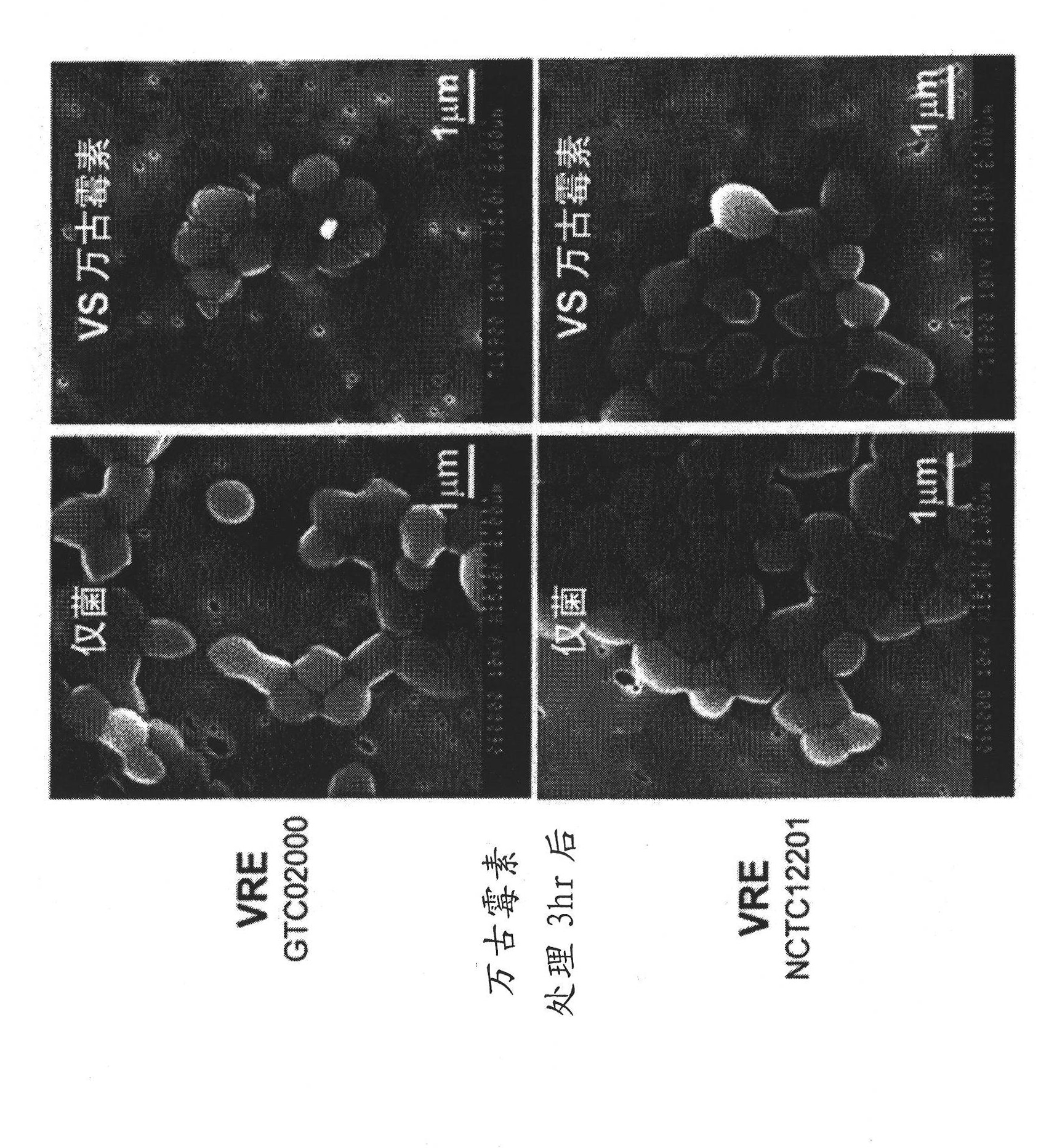
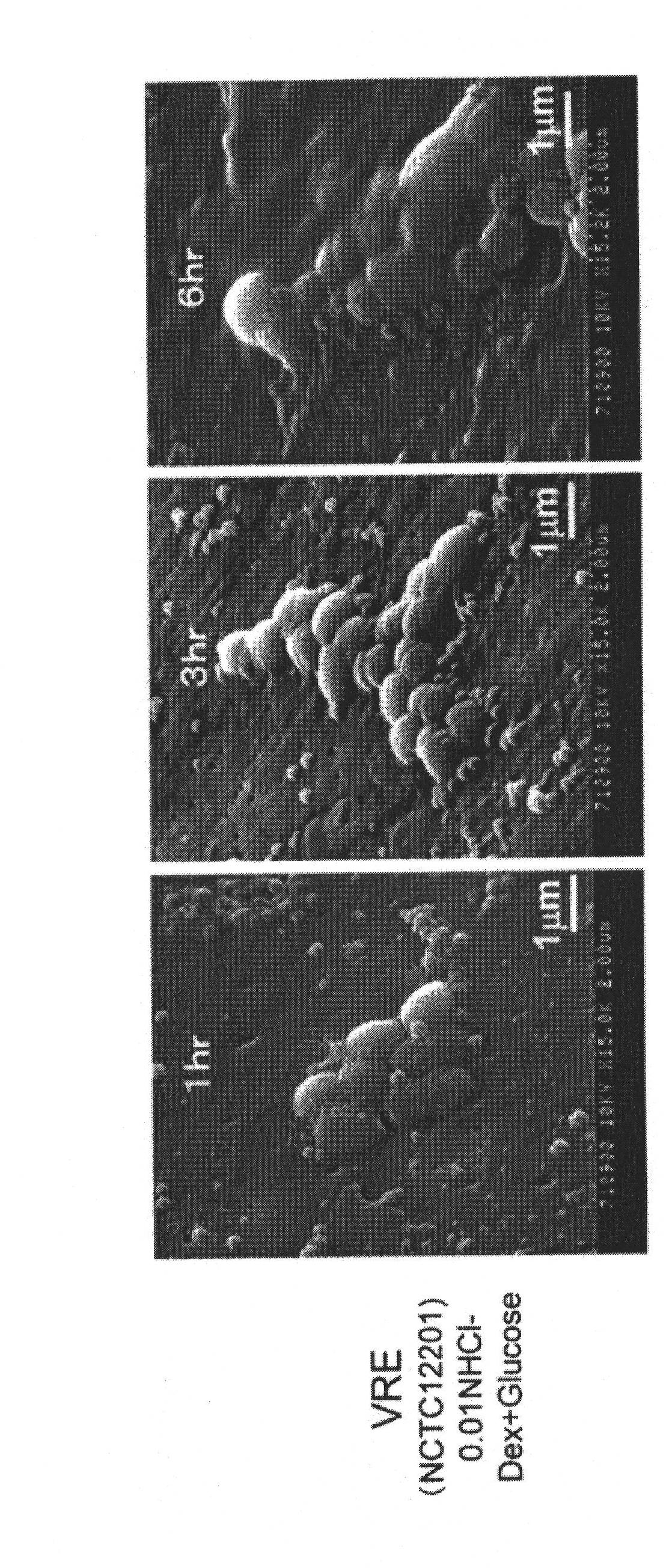
Antimicrobial agent for gram-positive bacteria
A gram-positive, antibacterial agent technology, applied in the field of antibacterial agents for gram-positive bacteria, can solve the problem of unknown antibacterial effect of microparticle monomers, etc., and achieve the effect of broadening the treatment path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0057] Production of Cyanoacrylate Polymer Particles
[0058] (dextran, dextran + glucose reaction system)
[0059]Cyanoacrylate polymer particles were produced using nBCA (Histoacryl (registered trademark), Braun Corporation, Melsungen, Germany) as a monomer. As the polymerization initiator-stabilizer, dextran alone or dextran+glucose was used. As the dextran, dextran (Dex70) having an average molecular weight of 70K was used.
[0060] Dissolve (1) 1 g of Dex70, or (2) 1 g of Dex70 and 5 g of glucose in 100 ml of hydrochloric acid. The concentration of hydrochloric acid is 0.05N, 0.01N, and 0.001N. While stirring each solution, 1.2 mL of nBCA (concentration in the reaction liquid: 1.2 v / v%) was added, and stirring was continued at room temperature for 2 hours (600 rpm) to carry out a polymerization reaction. 0.1N NaOH was added to the reaction liquid after polymerization to neutralize, and it stirred for 15 minutes after that. The reaction solution was filtered through a...
Embodiment 1
[0065] Antibacterial Activity of Cyanoacrylate Polymer Particles
[0066] (dextran, dextran + glucose reaction system)
[0067] The antibacterial activity (minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)) of the particles produced in Production Example 1 against various bacterial strains was investigated. As a positive control, ampicillin (ABPC) was used. The assay method is in accordance with the microfluidic dilution method (NCCLS). That is, APBC uses 256 μg / ml as a stock solution (1-fold dilution), and particles use 6.4 mg / ml as a stock solution (1-fold dilution), and each prepares a 12-level dilution series ranging from 2-fold dilution to 2048-fold dilution, and evaluates the antibacterial activity . Add these dilution series to a 96-well plate, adding strains to each well to reach 10 5 CFU / ml, incubate at 35°C for 18 hours, if turbidity is observed visually, it is regarded as the strain has developed, and the lowest concentration wh...
manufacture example 2
[0078] Production of Cyanoacrylate Polymer Particles
[0079] (glucose, ribose, lactose, trehalose reaction system)
[0080] Cyanoacrylate polymer particles were produced using nBCA (Histoacryl (registered trademark), Braun Corporation, Melsungen, Germany) as a monomer. As the polymerization initiator-stabilizer, glucose, ribose, lactose, trehalose are used.
[0081] Except using any one of 5 g of the above-mentioned monosaccharides and disaccharides as sugars, the same operation as in Production Example 1 was carried out to produce polycyanoacrylate particles (0.05NHCl-Glucose, 0.05NHCl-Ribose, 0.05NHCl-Ribose, 0.05NHCl- Lactose, 0.05NHCl-Trehalose, 0.01NHCl-Glucose, 0.01NHCl-Ribose, 0.01NHCl-Lactose, 0.01NHCl-Trehalose, 0.001NHCl-Glucose).
[0082] The obtained particles were subjected to He·Ne laser light scattering analysis using a commercially available measuring device (mentioned above), and the average particle diameter and ζ-potential were measured. The results are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com