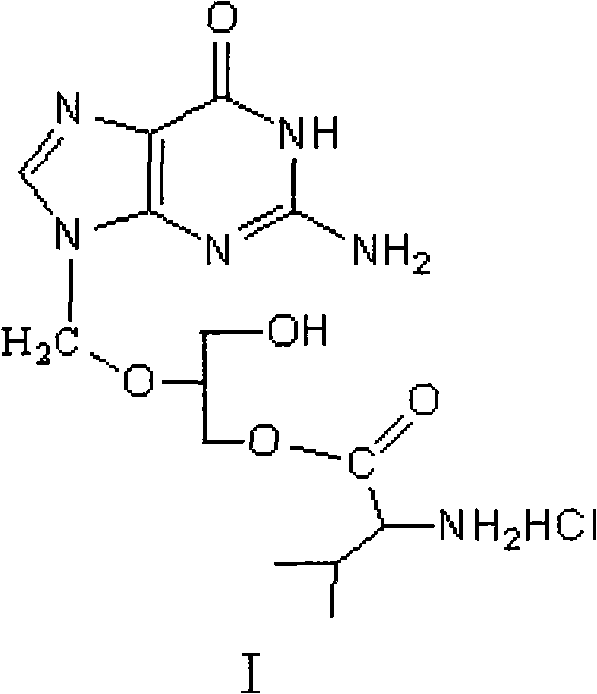
Method for preparing valganciclovir hydrochloride
A technology of valganciclovir hydrochloride and ganciclovir, applied in the field of medicine, can solve the problems of difficult separation of monoester and diester, unfavorable for industrialized production, unfavorable for industrialized production, etc., and achieves reduced post-processing difficulty and less impurities , the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of tert-butoxyphosphoryl dichloride
[0026] Add 30.7g of phosphorus oxychloride and 30ml of dichloromethane into a 100ml reaction flask, cool to below 0°C, add 22.2g of tert-butanol dropwise, then naturally warm up to room temperature and react for 3 hours, first evaporate the solvent under reduced pressure, and then 36.4 g of tert-butoxyphosphoryl dichloride were evaporated under reduced pressure with an oil pump, with a yield of 95.1%.
Embodiment 2
[0028] Synthesis of Ganciclovir Phosphate Monoester Ⅲ
[0029] Add 40.0g of tert-butoxyphosphoryl dichloride and 400ml of dichloromethane into a 1000ml reaction flask in Example 1, cool to below 0°C, and add 76.3g of ganciclovir under nitrogen protection, and then add 12ml of triethylamine , and then heated up to react at 20-50°C for 5 hours. The reaction was poured into ice water, the organic layer was separated, the aqueous layer was extracted once more with 200ml of dichloromethane, the combined organic layers were washed with water, the organic layer was dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. Vacuum dried to obtain ganciclovir monoester III 73.86g, yield 96.8%.
Embodiment 3
[0031] Synthesis of ganciclovir diester Ⅳ
[0032] Add 49g of ganciclovir monoester and 392mL of DMF in Example 2 into a 1000ml reaction flask, stir and cool down to 0-5°C, then add 53.9g of CBZ-L-Val, 2.1g of DMAP, 51.1g of DCC in sequence, and react at room temperature for 6 Hours later, cool down to 0°C for suction filtration, wash with 10mL of DMF, and dry the filtrate under reduced pressure at 110°C to a white solid, then add 490mL of methanol and stir to dissolve, slowly cool down to 10°C for suction filtration, and dry the filter cake at 80°C Cyclovir diester IV crude product: 74g, add 1295mL ethanol to the crude product, stir and heat up to 75°C to dissolve, slowly cool down to 0°C and filter with suction, and dry the filter cake at 80°C: 96.42g, yield: 88%, purity: 99.1 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com